Tell whether each of the following reactions favors reactants or products at equilibrium. (Assume that all reactants
Question:
Tell whether each of the following reactions favors reactants or products at equilibrium. (Assume that all reactants and products are soluble.)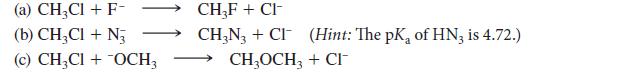
Transcribed Image Text:
(a) CH₂Cl + F- (b) CH3CI + N3 (c) CH₂Cl + OCH3 CH₂F+CI- CH₂N3+CI (Hint: The pK, of HN3 is 4.72.) CH3OCH3 + CI™
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 25% (4 reviews)
a Because fluoride ion is a stronger base than chloride ...View the full answer

Answered By

Muqadas Javed
I am a mentor by profession since seven years. I have been teaching on online forums and in universities. Teaching is my passion therefore i always try to find simple solution for complicated problems or task grasp them so that students can easily grasp them.I will provide you very detailed and self explanatory answers and that will help you to get good grade. I have two slogans: quality solution and on time delivery.
4.60+
24+ Reviews
144+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Tell wbether each of the following reactions favors reactants or products at equilibrium. (Assume that all reactants and products are soluble.) (a) CH3CI + I- CH3I + CI- (b) CH3CI + -OCH3 CH3OCH3 +...
-
Write a detailed paper on Trademark Law of the People's Republic of China
-
Assume the market for cat food is perfectly competitive. The cost curves for a typical cat food producer are depicted below. Indicate whether each of the following statements is true or false AND...
-
1) A survey of 200 public universities indicated that the 25th percentile of the yearly tuition cost of the universities was $4600 and the 75th percentile was $7100. The minimum value was $2000, the...
-
Describe the test for determining whether a governmental fund is a major fund. Describe the test for determining whether an enterprise fund is a major fund.
-
Jackson Corporation's bonds have 12 years remaining to maturity. Interest is paid annually, the bonds have a $1,000 par value, and the coupon interest rate is 8%. The bonds have a yield to maturity...
-
Compute dividend yield and explain its use in analysis. (p. 461) AppendixLO1
-
Metallica Can Opener Company is a subsidiary of Maltz Appliances, Inc. The can opener that Metallica produces is in strong demand. Sales this year are expected to be 1,000,000 units. Full plant...
-
Find the present value of $600 due in the future under each of these conditions: 9% nominal rate, semiannual compounding, discounted back 8 years. Round your answer to the nearest cent. $ 9% nominal...
-
What substitution and elimination products (if any) might be obtained when each of the following alkyl halides is treated with sodium methoxide in methanol? (a) Trans-1-bromo-3-methylcyclohexane (b)...
-
What product(s) are expected in the ethoxide-promoted b-elimination reaction of each of the following compounds? (a) 2-bromo-2,3-dimethylbutane (b) 1-chloro-1-methylcyclohexane
-
Assume that a virtual memory is managed using a buffer pool. The buffer pool contains five buffers and each buffer stores one block of data. Memory accesses are by block ID. Assume the following...
-
Compare the alternatives that Bergerac is considering for its decision. Include: Comparison of make versus buy option in the type of operation that Bergerac is looking to integrate. You do not need...
-
Let A, B, C and D be non-zero digits, such that CD is a two-digit positive integer. BCD is a three-digit positive integer generated by the digits B, C and D. ABCD is a four-digit positive integer...
-
1.) An aluminum tube is clamped with rigid plates using four bolts as shown. The nut on each bolt is tightened one turn from 'snug'. The thickness of the plate may be considered insignificant in this...
-
4.21 Case Study Competency IV.1RM Determine diagnosis and procedure codes and groupings according to official guidelines. Competency IV.1 Validate assignment of diagnostic and procedural codes and...
-
W.E.B Dubois taught the book called "The State" to his students at Atlanta University. Who wrote this book
-
Assume that square ABCD (Figure 2) has sides of length 1 and that E, F; G, and 11 are the midpoints of the sides. If the indicated pattern is continued indefinitely, what will be the area of the...
-
Recall that Chapter 8 described the binary search algorithm for finding a particular entry in an ordered list. The idea behind binary search is to begin looking in the exact center of the list. If...
-
Addition of HBr to a double bond with an ether (?OR) substituent occurs region specifically to give a product in which the ?Br and ?OR are bonded to the same carbon. Draw the two possible carbocation...
-
Alkyl halides can be reduced to alkanes by a radical reaction with tributyltin hydride, (C 4 H 9 ) 3 SnH, in the presence of light (h v ). Propose a radical chain mechanism by which the reaction...
-
Identify the reagents a?c in the following scheme: CH Br CH CH C CH
-
An underlying asset price is at 100, its annual volatility is 25% and the risk free interest rate is 5%. A European call option has a strike of 85 and a maturity of 40 days. Its BlackScholes price is...
-
Prescott Football Manufacturing had the following operating results for 2 0 1 9 : sales = $ 3 0 , 8 2 4 ; cost of goods sold = $ 2 1 , 9 7 4 ; depreciation expense = $ 3 , 6 0 3 ; interest expense =...
-
On January 1, 2018, Brooks Corporation exchanged $1,259,000 fair-value consideration for all of the outstanding voting stock of Chandler, Inc. At the acquisition date, Chandler had a book value equal...

Study smarter with the SolutionInn App


