Exactly 50 mL of a 0.0500 M solution of ethylamine, a base with K b = 1.1
Question:
Exactly 50 mL of a 0.0500 M solution of ethylamine, a base with Kb = 1.1 × 10-6, is titrated with 0.100 M HNO3. What is the pH at the equivalence point? Suggest a good indicator from Table 16.4 for this titration, and justify your selection.
Table 16.4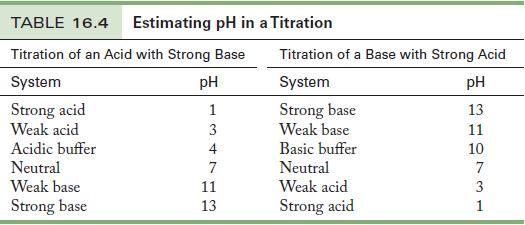
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
To find the pH at the equivalence point you need to consider what happens when you titrate a weak base with a strong acid At the equivalence point all ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Sketch the titration curve from Problem 123 by calculating the pH at the beginning of the titration, at one-half of the equivalence point, at the equivalence point, and at 5.0 mL beyond the...
-
Please write detailed roadmap/solution for all questions below. 1) An output of nmap search is shown below, a) Type the required terminal command and required parameters to obtain the shown output....
-
Harry Bhel carries a business as a sole proprietorship. During its 2022 fiscal period, its first year of operations, the business had cash sales of $123,000. It also has sales on account of $46,000,...
-
The following structure represents tetrahedral alkoxide ion intermediate formed by addition of a nucleophile to a carboxylic acid derivative. Identify the nucleophile, the leaving group, the starting...
-
After buying a computer system, Sim Thomas must decide whether to purchase (1) A complete maintenance (or service) policy at a cost of $500, which would cover all maintenance costs; (2) A partial...
-
how researchers used confidence intervals to study Uber and traffic fatalities
-
John Kyler is considering starting a Web-based educational business, e-Prep MBA. He plans to offer a short-course review of accounting for students entering MBA programs. The materials would be...
-
(Future value of an ordinary annuity). What is the future value of $480 per year for 10 years compounded annually at 10%? The future value of $480 per year for 10 years compounded annually at 10% is...
-
Write the chemical equilibria and expressions for the equilibrium constants for the ionizations of the following polyprotic acids. (a) Oxalic acid (b) Sulfurous acid
-
A 25.0-mL sample of 1.44 M NH 3 is titrated with 1.50 M HCl. Calculate the pH at the equivalence point. Choose an indicator from Table 16.4, and justify your choice. Table 16.4
-
Using your experience with Problem 8-82, specify an optimal bolt pattern for three bolts for the bracket in Problem 8-82 and size the bolts. Data in Problem 8-82, A cantilever is to be attached to...
-
The combined weight of the load and the platform is 200 lb, with the center of gravity located at G. If a couple moment of M = 900 lb ft is applied to link AB, determine the angular velocity of links...
-
Due In: 06:48:23 Questions Question 1 (4) O Question 2 (8) Question 2 of 2 A company sold $150,000 bonds and set up a sinking fund that was earning 8.5% compounded semi-annually to retire the bonds...
-
Find the point on the graph of f(x) = x which is closest to the point (6, 27). How close is the closest point?
-
Due to a crash at a railroad crossing, an overpass is to be constructed on an existing level highway. the existing highway has a design speed of 50 mi/h. The overpass structure is to be level,...
-
Finding Bone Density Scores. In Exercises 37-40 assume that a randomly selected subject is given a bone density test. Bone density test scores are normally distributed with a mean of 0 and a standard...
-
Ethanol (C2H5OH) is currently blended with gasoline as an automobile fuel. (a) Write a balanced equation for the combustion of liquid ethanol in air. (b) Calculate the standard enthalpy change for...
-
Provide examples of a situations in which environmental disruptions affected consumer attitudes and buying behaviors.
-
For the system in Fig. 11.26, compute the total head on the pump and the power delivered by the pump to the coolant. Figure 11.26 4 ft Flow 20 GPM 4.0 ft 4.0 ft L- 30 ft 1.0 ft 10 GPM Flow 18 ft 2.0...
-
The tank shown in Fig. 11.24 is to be drained to a sewer. Determine the size of new Schedule 40 steel pipe that will carry at least 400 gal/min of water at 80 F through the system shown. The total...
-
Water at 60F is to flow by gravity between two points, 2 mi apart, at the rate of 13 500 gal/min. The upper end is 130 ft higher than the lower end. What size concrete pipe is required? Assume that...
-
DETAILS 1. [-/1 Points) SMITHNM13 11.2.025. MY NOTES Convert the credit card rate to the APR. Oregon, 2% per month % Need Help? ReadIt Watch
-
Corom Stack Standard CALCULATOR PRINTER VERSION BACK NEXT Problem 13-02A a-c (Part Level Submission) Sheffield Corporation had the following stockholders' equity accounts on January 1, 2020: Common...
-
Suppose that you own 2,100 shares of Nocash Corp. and the company is about to pay a 25% stock dividend. The stock currently sells at $115 per share. a. What will be the number of shares that you hold...

Study smarter with the SolutionInn App


