A 25.0-mL sample of 1.44 M NH 3 is titrated with 1.50 M HCl. Calculate the pH
Question:
A 25.0-mL sample of 1.44 M NH3 is titrated with 1.50 M HCl. Calculate the pH at the equivalence point.
Choose an indicator from Table 16.4, and justify your choice.
Table 16.4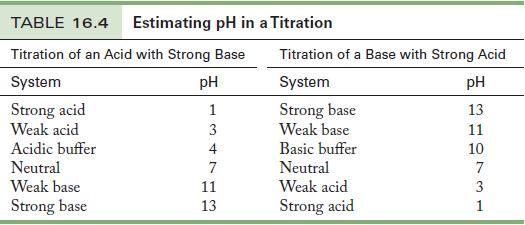
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
pH at the e...View the full answer

Answered By

Arun kumar
made more than four thousand assignments
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
A 10.0-mL solution of 0.300 M NH3 is titrated with a 0.100 M HCl solution. Calculate the pH after the following additions of the HCl solution: (a) 0.0 mL, (b) 10.0 mL, (c) 20.0 mL, (d) 30.0 mL, (e)...
-
Calculate the pH at the halfway point and at the equiva-lence point for each of the following titrations. a. 100.0 mL of 0.10 M HC7H5O2 (Ka = 6.4 105) titrated with 0.10 M NaOH b. 100.0 mL of 0.10 M...
-
Please write detailed roadmap/solution for all questions below. 1) An output of nmap search is shown below, a) Type the required terminal command and required parameters to obtain the shown output....
-
How do recruitment and selection practices contribute to high performance in an organization?
-
Predict the products of the following nucleophilic acyl substitutionreactions: (b) (a) NaOH NH3 H20 H (d) (c) Na* "OCH3 C CH3NH2 SCH3 "CH
-
Drink-At-Home, Inc. (DAH, Inc.), develops, processes, and markets mixes to be used in nonalcoholic cocktails and mixed drinks for home consumption. Mrs. Lee, who is in charge of research and...
-
When someone rates a group at 10 on the ANES feeling thermometer, thats a pretty serious sign of dislike. I make the claim that the population of African American, female strong Democrats aged 17 to...
-
Pastina Company manufactures and sells various types of pasta to grocery chains as private label brands. The company's fiscal year-end is December 31. The unadjusted trial balance as of December 31,...
-
Calculate the missing information for the instaliment loan that is being paid off early
-
Exactly 50 mL of a 0.0500 M solution of ethylamine, a base with K b = 1.1 10 -6 , is titrated with 0.100 M HNO 3 . What is the pH at the equivalence point? Suggest a good indicator from Table 16.4...
-
Chloropropionic acid, ClCH 2 CH 2 COOH, is a weak monoprotic acid with Ka = 7.94 10 -5 . Calculate the pH at the equivalence point in a titration of 10.00 mL of 0.100 M chloropropionic acid with...
-
Use regression analysis to fit a linear trend model to the data set. a. What is the estimated regression function? b. Interpret the R2 value for your model. c. Prepare a line graph comparing the...
-
Use the Comparison Theorem to determine whether the integral is convergent or divergent. L da
-
Problem 3 (2 scenarios) Scenario 1: Rocky Inc hired a new intern from CSU to help with year-end inventory. The intern computed the inventory counts at the end of 2020 and 2021. However, the intern's...
-
A CM reactor receives influent containing 10.0 mg/L of tracer for 2 h. Then tracer addition is terminated but the flow remains steady. The volume of the reactor is 10 L and the flow rate is 2 L / h....
-
Solve the given system of equations graphically by using a graphing calculator. y=5x x+y2=81 Find the solution with the smaller x-value. x= y= (Type an integer or a decimal rounded to one decimal...
-
I-The market for Sony's Playstation5 game console has changed from 2021 to 2023. With restrictions from the Covid-19 pandemic ending people are finding other entertainment options available such as...
-
Diethyl ether, C4H10O(l), a flammable compound that has long been used as a surgical anesthetic, has the structure CH3 CH2 O CH2 CH3 The complete combustion of 1 mol of C4H10O(l) to CO2(g) and...
-
Show that every group G with identity e and such that x * x = e for all x G is abelian.
-
What size of standard hydraulic copper tube from Appendix G.2 is required to transfer 0.06 m 3 /s of water at 80C from a heater where the pressure is 150 kPa to an open tank? The water flows from the...
-
Determine the required size of new Schedule 80 steel pipe to carry water at 160F with a maximum pressure drop of 10 psi per 1000 ft when the flow rate is 0.5 ft 3 /s.
-
A device designed to allow cleaning of walls and windows on the second floor of homes is similar to the system shown in Fig. 11.20. Determine the velocity of flow from the nozzle if the pressure at...
-
Diplomatic Security Service provides Airport Transportation and Surveillance Service to Foreign Diplomats in Guyana. The company has two support departments - Information Systems and Equipment...
-
Q1: A disparity of bargaining power between the parties to a contract may result in unfair terms but a court is not likely to consider the contract unconscionable. Group of answer choices a. True b....
-
Life Tool Manufacturing has a system in place to recall products that prove to be dangerous at some time after manufacture and distribution. This represents which element of the due care theory?...

Study smarter with the SolutionInn App


