Chloropropionic acid, ClCH 2 CH 2 COOH, is a weak monoprotic acid with Ka = 7.94
Question:
Chloropropionic acid, ClCH2CH2COOH, is a weak monoprotic acid with Ka = 7.94 × 10-5. Calculate the pH at the equivalence point in a titration of 10.00 mL of 0.100 M chloropropionic acid with 0.100 M KOH. Choose an indicator from Table 16.4 for the titration.
Explain your choice.
Table 16.4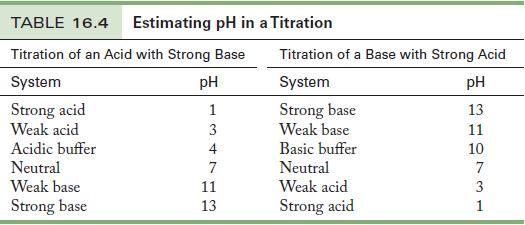
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
To calculate the pH at the equivalence point in a titration of a weak acid with a strong base we need to realize that at the equivalence point all the ...View the full answer

Answered By

Emel Khan
I have the ability to effectively communicate and demonstrate concepts to students. Through my practical application of the subject required, I am able to provide real-world examples and clarify complex ideas. This helps students to better understand and retain the information, leading to improved performance and confidence in their abilities. Additionally, my hands-on approach allows for interactive lessons and personalized instruction, catering to the individual needs and learning styles of each student.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Please write detailed roadmap/solution for all questions below. 1) An output of nmap search is shown below, a) Type the required terminal command and required parameters to obtain the shown output....
-
Ant venom contains formic acid (HCOOH; formica is the Latin word for ant). Suppose you are at a pharmaceutical company working on a quick antidote and need to estimate the pH at the stoichiometric...
-
What you thought you knew about the economics of healthcare and identify the significance (what it means to you professionally and/or personally). Please identify what more you want to learn in...
-
Describe Robertson Tool's business risk, making critical judgments. Consider the volatility of its revenues and operating expenses, therefore appraising the volatility of its EBIT. HINT: Consider the...
-
Rank the compounds in each of the following sets in order of their expected reactivity toward nucleophilic acylsubstitution: (a) , , , CH, HH. NH2 (b) CHC -CCI3, CHCICF3/2
-
Ruth Jones, a robust 50-year-old insurance adjuster living in the northern suburbs of Chicago, has been diagnosed by a University of Illinois cardiologist as having a defective heart valve. Although...
-
Run confidence intervals by state using the ANES Muslims feeling thermometer and report the 95% confidence intervals for the first five states: Alaska (AK), Alabama (AL), Arkansas (AR), Arizona (AZ),...
-
Quattro, Inc. has the following mutually exclusive projects available. The company has historically used a 4-year cutoff for projects. The required return is 11 percent. a. Compute the payback for...
-
Consider a property with NOI equal to $40,000, there are $5,000 in capital expenditures there is $20,000 interest-only loan at 8 percent annual interest, the depreciable cost basis of the residential...
-
A 25.0-mL sample of 1.44 M NH 3 is titrated with 1.50 M HCl. Calculate the pH at the equivalence point. Choose an indicator from Table 16.4, and justify your choice. Table 16.4
-
A chemist is developing a titration analysis for lactic acid. Lactic acid is a monoprotic acid with K a = 8.4 10 -4 . Calculate the pH at the equivalence point of a titration of 100 mL of 0.100 M...
-
Refer to the list of warnings on pages 527 528. Explain which ones should be of concern if the sample size(s) for a test are large. From this discussion, you should realize that you cant simply rely...
-
3 Below is financial information for December Inc., which manufactures a single product: 4 5 5 7 3 #units produced October Low activity November High activity 7,000 11,000 Cost of goods manufactured...
-
(b) The satellite's booster rockets fire and lift the satellite to a higher circular orbit of radius 2R1. The satellite follows the path shown in the diagram below, moving a total distance S during...
-
D. An airplane flies at a speed of 250 kilometers per hour (kph) at an altitude of 3000 m. Assume the transition from laminar to turbulent boundary layers occurs at critical Reynolds Number, RE cr, =...
-
1. (35 points) by Qet = QoQ = Q0Q2 N!37 N/A (1-B, (r), where A- B(T) V * 4R (e-(R)/KT - 1) R dR is the second virial coefficient. The classical partition function for an imperfect gas comprising N...
-
es Farm has 28 employees who are paid biweekly. The payroll register showed the following payroll deductions for the pay period ending March 23, 2021. Gross Pay 85,950.00 EI Premium Income Taxes...
-
Gasoline is composed primarily of hydrocarbons, including many with eight carbon atoms, called octanes. One of the cleanest-burning octanes is a compound called 2,3,4- trimethylpentane, which has the...
-
The electric field due to a line charge is given by where l is a constant. Show that E is solenoidal. Show that it is also conservative. E =
-
Turpentine at 77F is flowing from A to B in a 3-in coated ductile iron pipe. Point B is 20 ft higher than point A and the total length of the pipe is 60 ft. Two 90 long-radius elbows are installed...
-
Water at 15C is flowing downward in a vertical tube 7.5 m long. The pressure is 550 kPa at the top and 585 kPa at the bottom. A ball-type check valve is installed near the bottom. The hydraulic tube...
-
In a processing plant, ethylene glycol at 77F is flowing in a 6-in coated ductile iron pipe having a length of 5000 ft. Over this distance, the pipe falls 55 ft and the pressure drops from 250 psig...
-
1. (A nice inharitage) Suppose $1 were invested in 1776 at 3.3% interest compounded yearly a) Approximatelly how much would that investment be worth today: $1,000, $10,000, $100,000, or $1,000,000?...
-
Why Should not the government subsidize home buyers who make less than $120K per year. please explain this statement
-
Entries for equity investments: 20%50% ownership On January 6, 20Y8, Bulldog Co. purchased 25% of the outstanding common stock of $159,000. Gator Co. paid total dividends of $20,700 to all...

Study smarter with the SolutionInn App


