Explain why the algebraic expression can be used for dilution problems but not for titration calculations. V
Question:
Explain why the algebraic expression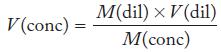
can be used for dilution problems but not for titration calculations.
Transcribed Image Text:
V (conc) = M(dil) × V(dil) M(conc)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
In a dilution problem the moles in the dilute ...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
KYC's stock price can go up by 15 percent every year, or down by 10 percent. Both outcomes are equally likely. The risk free rate is 5 percent, and the current stock price of KYC is 100. (a) Price a...
-
Dilution of the Maalox (milk of magnesia with magnesium hydroxide) Solution 1. Record the brand and %m/v Mg(OH) 2 in your Maalox antacid or generic form. Note that some brands contain Al(OH) 3 . 2....
-
Read the comment letter below written by Micron's CFO to the FASB dated June 30, 2004 and answer the following question. Director of Major Projects Financial Accounting Standards Board 401...
-
What is your debt payments-to-income ratio if your debt payments total $684 and your net income is $2,000 per month?
-
It is proposed to use 113Cd48 as an attenuator in a nuclear pile. Calculate the thickness required to attenuate a neutron beam to 0.01% of its original intensity it if has a density of 8.7 x 103 kg...
-
The cost of an asset as well as its accumulated depreciation is removed when an asset is sold. True or False
-
What do you recommend that Hoi do to improve the companys cash flow? LO.1
-
The manager of a $20 million portfolio of domestic stocks with a beta of 1.10 would like to begin diversifying internationally. He would like to sell $5 million of domestic stock and purchase $5...
-
At December 31, 2018, the accounting records of Hercules Manufacturing, Inc. contain the following items: Accounts Payable Land Building Notes Payable Retained Earnings $ 12,000 Accounts Receivable $...
-
Rob is selling his house in Tucson. Jim, his agent, has found a buyer, Sid. Sid signs a contract in which he agrees to buy the house for $250,000 but the document provides that "this sale is...
-
What volume of 0.20 M HNO 3 is needed to react completely with 37 g Ca(OH) 2 ? Strategy We use the same four steps as for all stoichiometry problems. Write the balanced equation, calculate the amount...
-
Calculate the mass of AgCl that forms in the reaction of 0.500 L of 1.30 M CaCl 2 with excess AgNO 3 .
-
You work for Midstates Solar Power. A manager asked you to determine which of the following two machines will have the lower (a) Capital recovery (b) Equivalent annual total cost. Machine Semi2 has a...
-
Let $N$ be a positive integer. Consider the relation $\circledast$ among pairs of integers $r, s \in \mathbb{Z}$ defined as $r \circledast s$ when $r-s$ is an integer multiple of $N$. Prove that...
-
Draw a circuit diagram for a typical home hair dryer. To which form (or forms) of energy is electric potential energy converted when you use the dryer?
-
Draw a vector field diagram for particles carrying charges \(+2 q\) and \(-q\) separated by a distance \(r\). Comment on the significance of the vector diagram.
-
(a) Show that the Jones matrix of a polarization analyzer set at angle \(\alpha\) to the \(X\)-axis is given by \[ \underline{\mathbf{L}}(\alpha)=\left[\begin{array}{cc} \cos ^{2} \alpha & \sin...
-
Let \(\mathbf{V}(t)\) be a linearly filtered complex-valued, wide-sense stationary random process with sample functions given by \[ \mathbf{v}(t)=\int_{-\infty}^{\infty} \mathbf{h}(t-\tau)...
-
What is an NSF cheque?
-
Three successive resonance frequencies in an organ pipe are 1310, 1834, and 2358 Hz. (a) Is the pipe closed at one end or open at both ends? (b) What is the fundamental frequency? (c) What is the...
-
Why didnt Joule change his experiment to make C surroundings /C system approx = 0.001 to increase the sensitivity of the apparatus?
-
Why does the relation C P > C V always hold for a gas? Can C P < C V be valid for a liquid?
-
Why can q V be equated with a state function if q is not a state function?
-
Accounting changes fall into one of three categories. Identify and explain these categories and give an example of each one.
-
Machinery is purchased on May 15, 2015 for $120,000 with a $10,000 salvage value and a five year life. The half year convention is followed. What method of depreciation will give the highest amount...
-
Flint Corporation was organized on January 1, 2020. It is authorized to issue 14,000 shares of 8%, $100 par value preferred stock, and 514,000 shares of no-par common stock with a stated value of $2...

Study smarter with the SolutionInn App


