For each of the following molecules, complete the Lewis structure and use the VSEPR model to determine
Question:
For each of the following molecules, complete the Lewis structure and use the VSEPR model to determine the bond angles around each central atom. Note that the drawings are only skeleton structures and may depict the angles incorrectly.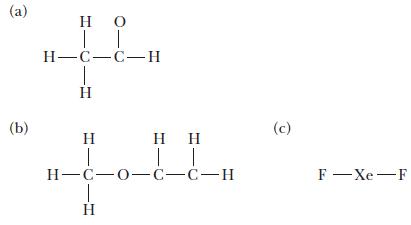
Transcribed Image Text:
(a) (b) IT H=C=C= H IT H-C-0-C-C-H (c) F - Xe-F
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
a H O I HCCH I H 1095 arou...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
For each of the following molecules, complete the Lewis structure and use the VSEPR model to determine the bond angles around each central atom. Note that the drawings are only skeleton structures...
-
For each of the following molecules, complete the Lewis structure and use the VSEPR model to determine the bond angles around each central atom. Note that the drawings are only skeleton structures...
-
For each of the following molecules, complete the Lewis structure and use the VSEPR model to determine the bond angles around each central atom. Note that the drawings are only skeleton structures...
-
Ratio Computation and Analysis; Liquidity) as loan analyst for Madison Bank, you have been presented the following information. Each of these companies has requested a loan of $50,000 for 6 months...
-
Industry-Specific Ratios Specialized ratios are sometimes used in specific industries, For example, the so-called book-to-bill ratio is closely watched for semiconductor manufacturers. A ratio of .93...
-
Which one of the following statements is false? a. Facility support costs should not be allocated over each division. b. Facility support costs include direct labor and direct materials. c....
-
Identify a changing perspective on performance? lo1
-
Consider again the choice between outsourcing and in-house assembly of HomeNet discussed in Section 8.3 and analyzed in Table 8.6. Suppose, however, that the upfront cost to set up for in-house...
-
11. Which of the following is TRUE of a transaction that involves after sales service? It: a. is no longer regarded as revenue b. means that the revenue is spread over the service period c. is always...
-
Indicate which of the following molecules are polar. Draw the molecular structure of each polar molecule, including the arrows that indicate the bond dipoles and the molecular dipole moment. (a) NF 3...
-
The molecular orbital diagram of NO shown in Figure 10.47 also applies to OF - . Draw the complete molecular orbital diagram for OF - . What is the OF bond order? Figure 10.47 Energy Atomic orbitals...
-
Which of the following statements is not true of a phasor? (a) It may be a scalar or a vector. (b) It is a time-dependent quantity. (c) A phasor Vs may be represented as . (d) It is a complex...
-
Sams old friend Dot is considering setting up a business offering historical boating trips along the River Thames. Dot thinks that she may be able to make a good living out of this. She has carried...
-
Arrow Industries employs a standard cost system in which direct materials inventory is carried at standard cost. Arrow has established the following standards for the direct costs of one unit of...
-
Explain the financial effect (increase, decrease, or no effect) of each of the following transactions on stockholders' equity: a. Purchased supplies for cash. b. Paid an account payable. c. Paid...
-
What type of account-asset, liability, stockholders' equity, dividend, revenue, or expense-is each of the following accounts? Indicate whether a debit entry or a credit entry increases the balance of...
-
Is it possible for an accounting transaction to only affect the left side of the accounting equation and still leave the equation in balance? If so, provide an example.
-
A band from a column eluted at 0.66 mL/min has a width at half-height, w1/2, of 39.6 s. The sample was applied as a sharp plug with a volume of 0.40 mL, and the detector volume is 0.25 mL. Find the...
-
Suppose you won a financial literacy competition and are given FJS10000 to invest, with the condition that investment can be done either in, i) Invest in Unit trust of Fiji or Invest in Fijian...
-
The shaft is supported by smooth journal bearings at A and B that only exert vertical reactions on the shaft. Determine its smallest diameter d if the allowable bending stress is Ï allow = 180...
-
The axle of the freight car is subjected to a wheel loading of 20 kip. If it is supported by two journal bearings at C and D, determine the maximum bending stress developed at the center of the axle,...
-
The strut on the utility pole supports the cable having a weight of 600 lb. Determine the absolute maximum bending stress in the strut if A, B, and C are assumed to be pinned. 2 in. -4 ft- -2 ft C01...
-
Caspian Sea Drinks needs to raise $74.00 million by issuing additional shares of stock. If the market estimates CSD will pay a dividend of $2.69 next year, which will grow at 3.45% forever and the...
-
i need help in B and C Integrative Case 5-72 (Algo) Cost Estimation, CVP Analysis, and Decision Making (LO 5-4.5.9) Luke Corporation produces a variety of products, each within their own division....
-
Relate PSA (Public Securities Association) speed to the average life of a MBS. Describe the PSA measure and discuss which MBS would have the greater average life, one with a PSA of 100 or one with a...

Study smarter with the SolutionInn App


