The molecular orbital diagram of NO shown in Figure 10.47 also applies to OF - . Draw
Question:
The molecular orbital diagram of NO shown in Figure 10.47 also applies to OF-. Draw the complete molecular orbital diagram for OF-. What is the OF bond order?
Figure 10.47 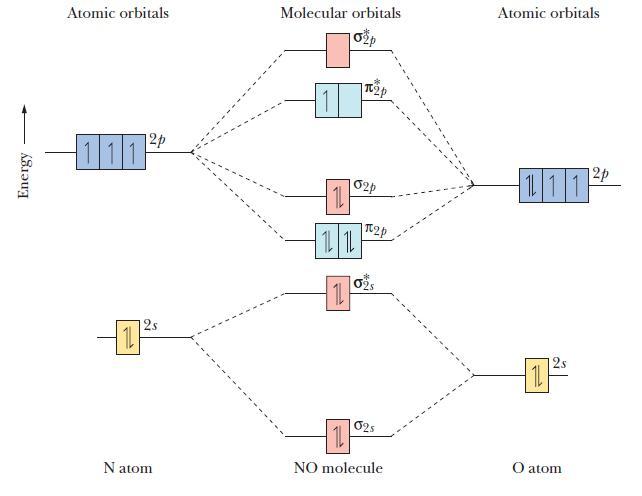
Transcribed Image Text:
Energy Atomic orbitals 12p 111 12s Natom Molecular orbitals 11 1L 1L 11 p 02p 2p 02S 025 11 NO molecule Atomic orbitals 111 11 2 12p O atom
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
While I cannot draw and provide you the complete molecular orbital diagram for OF here I can explain how to construct one based on the provided diagra...View the full answer

Answered By

Emel Khan
I have the ability to effectively communicate and demonstrate concepts to students. Through my practical application of the subject required, I am able to provide real-world examples and clarify complex ideas. This helps students to better understand and retain the information, leading to improved performance and confidence in their abilities. Additionally, my hands-on approach allows for interactive lessons and personalized instruction, catering to the individual needs and learning styles of each student.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
The molecular orbital diagram of NO shown in Figure 10.47 also applies to CO. Draw the complete molecular orbital diagram for CO. What is the CO bond order? Figure 10.47 Energy Atomic orbitals 12p...
-
The molecular orbital diagram of NO shown in Figure 10.47 also applies to the following species. Write the molecular orbital electron confi guration of each, indicating the bond order and the number...
-
The nitrosyl ion, NO + , has an interesting chemistry. Assume the molecular orbital diagram shown in Figure 9.16 applies to NO + . (a) Is NO + diamagnetic or paramagnetic? If paramagnetic, how many...
-
Northland Corporation is a small information-systems consulting firm that specializes in helping companies implement standard sales-management software. The market for Northalndss services is very...
-
Du Pont Identity Why is the Du Pont identity a valuable tool for analyzing the performance of a firm discuss the types of information ii reveals compared to ROE considered by itself.
-
Refer to the data in PE 18-7. Using the following additional information, compute the cost of materials to be used in the coming period. (Assume no beginning or ending direct materials inventory.)...
-
Consider the probability distribution shown here. a. Find p, n2 , and a. b. Find the sampling distribution of T for random samples of n = 2 measurements from this distribution by listing all possible...
-
What are the real economic impacts and long-term effects of trade sanctions? Assume that the United States imposes punishingly high tariffs of 100 percent on Japanese cars. Immediate costs might be...
-
Both parts please
-
For each of the following molecules, complete the Lewis structure and use the VSEPR model to determine the bond angles around each central atom. Note that the drawings are only skeleton structures...
-
The delocalized bonding that describes O 3 also applies to NO 2 . Draw the delocalized molecular orbital for NO 2 .
-
What should Avery Mitchell do next? What information should he ask the consultants for before accepting their proposal? What project planning tools would you suggest Avery ask the consultants to use...
-
The following information is available for two different types of businesses for the 2011 accounting period. Dixon Consulting is a service business that provides consulting services to small...
-
Marino Basket Company had a \(\$ 6,200\) beginning balance in its Merchandise Inventory account. The following information regarding Marino's purchases and sales of inventory during its 2011...
-
On March 6, 2011, Bob's Imports purchased merchandise from Watches Inc. with a list price of \(\$ 31,000\), terms \(2 / 10, n / 45\). On March 10, Bob's returned merchandise to Watches Inc. for...
-
The following events apply to Tops Gift Shop for 2012, its first year of operation: 1. Acquired \(\$ 45,000\) cash from the issue of common stock. 2. Issued common stock to Kayla Taylor, one of the...
-
Indicate whether each of the following costs is a product cost or a period (selling and administrative) cost. a. Transportation-in. b. Insurance on the office building. c. Office supplies. d. Costs...
-
A chromatographic band has a width, w, of 4.0 mL and a retention volume of 49 mL. What width is expected for a band with a retention volume of 127 mL? Assume that the only band spreading occurs on...
-
The trade-off theory relies on the threat of financial distress. But why should a public corporation ever have to land in financial distress? According to the theory, the firm should operate at the...
-
The boat has a weight of 2300 lb and a center of gravity at G. If it rests on the trailer at the smooth contact A and can be considered pinned at B, determine the absolute maximum bending stress...
-
Determine the absolute maximum bending stress in the 1.5-in.-diameter shaft. The shaft is supported by a thrust bearing at A and a journal bearing at B. 400 Ib 300 lb 12 in 18 in. 15 in.
-
Determine the smallest allowable diameter of the shaft. The shaft is supported by a thrust bearing at A and a journal bearing at B. The allowable bending stress is Ï allow = 22 ksi. 400 lb 300...
-
The following amounts were reported on the December 31, 2022, balance sheet: Cash $ 8,000 Land 20,000 Accounts payable 15,000 Bonds payable 120,000 Merchandise inventory 30,000 Retained earnings...
-
Sandhill Co. issued $ 600,000, 10-year, 8% bonds at 105. 1.Prepare the journal entry to record the sale of these bonds on January 1, 2017. (Credit account titles are automatically indented when the...
-
Based on the regression output (below), would you purchase this actively managed fund with a fee of 45bps ? Answer yes or no and one sentence to explain why.

Study smarter with the SolutionInn App


