The molecular orbital diagram of NO shown in Figure 10.47 also applies to the following species. Write
Question:
The molecular orbital diagram of NO shown in Figure 10.47 also applies to the following species. Write the molecular orbital electron confi guration of each, indicating the bond order and the number of unpaired electrons.
(a) LiBe+
(b) CO+
(c) CN-
(d) OF
Figure 10.47 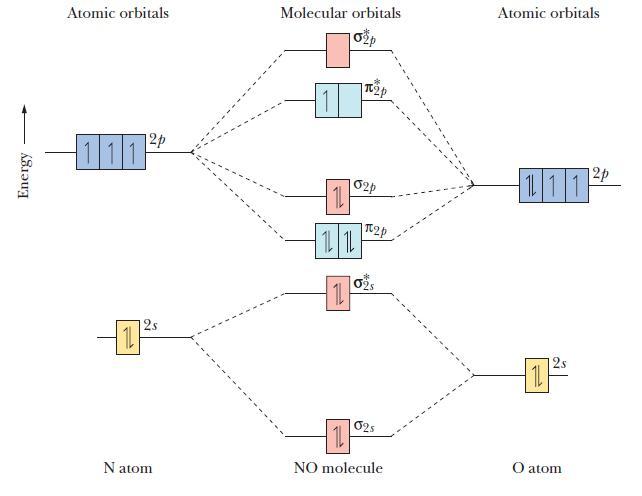
Transcribed Image Text:
Energy Atomic orbitals 12p 111 12s Natom Molecular orbitals 11 1L 1L 11 p 02p 2p 02S 025 11 NO molecule Atomic orbitals 111 11 2 12p O atom
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
a 00 2p02 bond order is 25 with one unpaire...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
The molecular orbital diagram of NO shown in Figure 10.47 also applies to CO. Draw the complete molecular orbital diagram for CO. What is the CO bond order? Figure 10.47 Energy Atomic orbitals 12p...
-
The molecular orbital diagram of NO shown in Figure 10.47 also applies to OF - . Draw the complete molecular orbital diagram for OF - . What is the OF bond order? Figure 10.47 Energy Atomic orbitals...
-
The nitrosyl ion, NO + , has an interesting chemistry. Assume the molecular orbital diagram shown in Figure 9.16 applies to NO + . (a) Is NO + diamagnetic or paramagnetic? If paramagnetic, how many...
-
On January 1, 2012, Albert invested $1,000 at 6 percent interest per year for three years. The CPI (times 100) on January 1, 2012, stood at 100. On January 1, 2013, the CPI was 105; on January 1,...
-
In the network in figure, the switch opens at t = 0. Use Laplace transform to find iL(t) for t >0. 0.5 F 1 = 0 1 A
-
Multiple-Step Income Statement Gaynor Corporations partial income statement is as follows: Sales . . . . . . . . . . . . . . . . . . . . . . . . . . . . $1,200,000 Cost of sales . . . . . . . . . . ....
-
Random samples of size n1 = 55 and n2 = 65 were drawn from populations 1 and 2, respectively. The samples yielded p, = .7 and ,E, = .6. Test HI < (p, - p,) = 0 against H,: (pl - p,) > 0 using a =...
-
The Coca-Cola Company and PepsiCo, Inc. Instructions Go to the books companion website and use information found there to answer the following questions related to The Coca-Cola Company and PepsiCo,...
-
Koontz Company manufactures two models of industrial componentsa Basic model and an Advanced Model. The company considers all of its manufacturing overhead costs to be fixed and it uses plantwide...
-
Assuming that the molecular orbital diagram shown in Figure 10.40 is correct for heteronuclear diatomic molecules containing elements that are close to each other in the periodic table, write a...
-
Aspartame is a compound that is 200 times sweeter than sugar and is used extensively (under the trade name NutraSweet) in diet soft drinks. The skeleton structure of the atoms in aspartame is H-0. ....
-
Identify the factors that determine the proportion of the value of property held in joint tenancy with the right of survivorship that will be included in a decedent's gross estate.
-
Part 2 One Stop Electrical Shop are merchandisers of household fixtures & fittings. The business began the last quarter of 2020 (October to December) with 25 Starburst Wall Clocks at a total cost of...
-
A species of butterfly has three subspecies A, B, and C. A scientist is trying to classify observed. specimens into these subspecies based on the color of their wings, which can be blue, green, pink,...
-
Stock during the year were sold for $8 per share. On December 31 , Portland had no remaining treasury stock. Required: Prepare the necessary journal entries to record any transactions associated with...
-
2) 20 pts. A 2-kg block rests on a wedge that has a coefficient of friction between the wedge and block of 0.3. The system accelerated to the right. Determine the maximum acceleration of the system...
-
ABC Ltd. is concerning about its poor performance and considering whether or not dropping the production and sells of product R, which incurs losses of Birr 4000. Additional information: The salaries...
-
The space shuttle's expendable booster engines derive their power from solid reactants: 6NH4+ ClO-4 (s) + 10Al(s) 3N2(g) + 9H2O(g) + 5Al2O3(s) + 6HCI(g) (a) Find the oxidation numbers of the...
-
Write a program to move a signed number from smaller register to bigger register. Hint: movzx ax, bl Topic: Data Related Operators and Directives in assembly language
-
The column is built up by gluing the two boards together. Determine the maximum normal stress on the cross section when the eccentric force of P = 50 kN is applied. 150 mm 250 mm 75 mm 150 mm 50 mm...
-
The steel bracket is used to connect the ends of two cables. If the applied force P = 1.50 kip, determine the maximum normal stress in the bracket. Assume the bracket is a rod having a diameter of...
-
The steel bracket is used to connect the ends of two cables. If the allowable normal stress for the steel is sallow = 30 ksi, determine the largest tensile force P that can be applied to the cables....
-
How do warehouses and distribution centers differ? What is cross-docking and why might a company choose to cross-dock a product? What kinds of products can be delivered electronically? What kinds...
-
Strawberry Inc. has historically been an all-equity firm. The analyst expects EBIT to be $1.5B in perpetuity starting one year from now. The cost of equity for the company is 11.5% and the tax rate...
-
Guzman company received a 60- day, 5 % note for 54,000 dated July 12 from a customer on account. Determine the due date on note. Determine the maturity value of the note and journalize the entry of...

Study smarter with the SolutionInn App


