For each of the reactions, calculate E from the table of standard potentials, and state whether the
Question:
For each of the reactions, calculate E° from the table of standard potentials, and state whether the reaction is spontaneous as written or spontaneous in the reverse direction under standard conditions.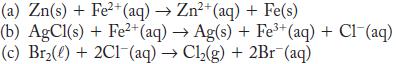
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
a E 136 V spontaneous in the ...View the full answer

Answered By

Muhammad Haroon
More than 3 years experience in teaching undergraduate and graduate level courses which includes Object Oriented Programming, Data Structures, Algorithms, Database Systems, Theory of Automata, Theory of Computation, Database Administration, Web Technologies etc.
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
For each of the reactions, calculate E from the table of standard potentials, and state whether the reaction is spontaneous as written or spontaneous in the reverse direction under standard...
-
Calculate the potential for each of the voltaic cells in Exercise 18.44 when the concentrations of the soluble species and gas pressures are as follows: Exercise 18.44 For each of the reactions,...
-
(a) LEP Table 12-2: Exothermic Reaction with Heat Exchange Download the Polymath, MATLAB, Python, or Wolfram codes for the algorithm and data given in Table T12-2 for the exothermic gas phase...
-
Which of the following options are available for creating a policy in Qualys Policy Compliance? (Choose three) A, Create from Host B, Create from Scratch C, Import from Library D, Import from CSV File
-
Growers use giant fans to prevent grapes from freezing when the effective sky temperature is low. The grape, which may be viewed as a thin skin of negligible thermal resistance enclosing a volume of...
-
Virginia Tech operates its own power generating plant. The electricity generated by this plant supplies power to the university and to local businesses and residences in the Blacksburg area. The...
-
(Computation of Pension Expense) Rebekah Company provides the following information about its defined benefit pension plan for the year 2011. Service cost $ 90,000 Contribution to the plan 105,000...
-
Explain how a U.S. companys commitment to purchase inventory with settlement in foreign currency (FC) might become less attractive over time and how adverse effects on earnings could be reduced.
-
Consider the following scenario. For a weekly pay period, you know the following information about the payroll at Fort Kent Industries. Friday is a mandatory holiday. Chris receives an annual salary...
-
Use the data from the table of standard reduction potentials in Appendix H to calculate the standard potential of the cell based on each of the following reactions. In each case, state whether the...
-
Two electrodes are immersed in a 1 M HBr solution. One of the electrodes is a silver wire coated with a deposit of AgBr(s). Th e second electrode is a platinum wire in electrical contact with a...
-
Evaluate the following integrals. x cos 2 (x) 2 dx
-
How do socio-cognitive mechanisms, such as social identity theory and self-categorization theory, contribute to the formation and maintenance of organizational culture ?
-
How do you Sales Forecast and an Expense forecast for future years?
-
2. Do you really think the Bono case described in Ch. 2 is a genuine ethical conflict? Explain. 6. Describe the ethical issue in the Siemens case
-
How do I calculate using the SPC method if my key metric is time
-
Labor Standards: Where Do They Belong on the International Trade Agenda? Author(s): Drusilla K. Brown Link. https://viu.summon.serialssolutions.com/?#!/search?....
-
Energy is required to remove two electrons from Ca to form Ca2+ and is required to add two electrons to O to form O2-. Why, then, is CaO stable relative to the free elements?
-
When a company has a contract involving multiple performance obligations, how must the company recognize revenue?
-
(a) Suppose a tire rolls without slipping on a horizontal road. Explain the role friction plays in this motion. What two surfaces are involved in this frictional force? Is it static friction or...
-
You are a newly graduated astronaut preparing for your first trip into space. Plans call for your spacecraft to reach a velocity of 500 m/s after 2.4 min. If your mass is 75 kg, what force will be...
-
The lower piece of silk in Figure 3.20 is acted on by two forces, +T 2 at the upper end and -T 2 at the lower end. These two forces are equal in magnitude and opposite in direction. Are they an...
-
4 Exercise 9-6 (Algo) Lower of cost or market [LO9-1) 75 Tatum Company has four products in its inventory. Information about the December 31, 2021, Inventory is as follows: oints Product Total Cost...
-
A real estate investment is expected to return to its owner $3,500 per year for 16 years after expenses. At the end of year 16, the property is expected to be sold for $49,000. Assuming the required...
-
You borrowed $15,000 for buying a new car from a bank at an interest rate of 12% compounded monthly. This loan will be repaid in 48 equal monthly installments over four years. Immediately after the...

Study smarter with the SolutionInn App


