From each pair of complexes, select the one that has the greater crystal field splitting.
Question:
From each pair of complexes, select the one that has the greater crystal field splitting.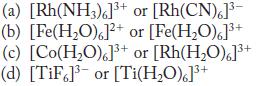
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
The crystal field splitting in transition metal complexes is dependent on the ligands that surround ...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
From each pair of complexes, select the one that has the greater crystal field splitting.
-
For each pair of complexes listed, select the one that has the larger value of . Strategy Consult the spectrochemical series, and also consider the charge on the metal and the row in which the metal...
-
Susan, Peter, and Mamamia hold 30% of the shares in AV COVID-19 Pty Ltd and the rest of the shares are held by Antonio and Anthony, 5% each. The company's constitution states that all the company's...
-
In 2017, Barlow moved from Chicago to Miami to start a new job, incurring costs of $1,200 to move household goods and $2,500 in temporary living expenses. Barlow was not reimbursed for any of these...
-
Section 10.5 used the predicates Link and Link Text to describe connections between web pages. Using the In Tag and Get Page predicates, among others, write definitions for Link and Link Text.
-
Consider the futures contract written on the S&P 500 index and maturing in 6 months. The interest rate is 3% per 6-month period, and the future value of dividends expected to be paid over the next 6...
-
Why does rebalancing work as a surrogate for misclassification costs? Use the following information for Exercises 2744. Suppose that our client is a retailer seeking to maximize revenue from a direct...
-
The following data are for Ernst Company. Note: All inventory is purchased on account, and Accounts Payable relates only to the purchase of inventory. Instructions: Compute the following: 1. The...
-
10% Preference Share 6,00,000 Investment in Shares in capital 80,000 shares Ltd. at Cost 3,24,000 of Rs. 7.5 each Less: Reduced (11 500) 3,12,500 Reserve and surplus Stock 2,48,000 Capital reserve...
-
For each d electron configuration, state the number of unpaired electrons expected in octahedral complexes. Give an example complex for each case. (Two answers are possible for some of these cases.)...
-
What physical property is different for two enantiomers?
-
Under what circumstances does the x 2 distribution provide an appropriate characterization of the sampling distribution of the Kruskal-Wallis H-statistic?
-
Given the following differential equation, dydx = sin ( x + y ) Find the following: ( a ) The substitution u = ( b ) The transformed differential equation dudx = ( c ) The implicit solution, given...
-
Consider the following type declarations TYPE Alinteger; A2 pointer to float; A3 pointer to integer; T1 structure (x: integer; } T2 structure (x: A1; next pointer to integer; } b float; } a :...
-
https://www.viddler.com/embed/82b62f65 Questions: How do companies decide where to locate their facilities? Why has just-in-time inventory control become a dominant production process used in the...
-
Adjusting Entries for Interest At December 31 of Year 1, Portland Corporation had two notes payable outstanding (notes 1 and 2). At December 31 of Year 2, Portland also had two notes payable...
-
We want to get an idea of the actual mass of 235U involved in powering a nuclear power plant. Assume that a single fission event releases 200 MeV of thermal energy. A 1,000 MWe electric power plant...
-
A manufacturing process is designed to produce an electronic component for use in small portable television sets. The components are all of standard size and need not conform to any measurable...
-
Consider a closed, rigid tank with a volume of 0.8L, filled with cold water initially at 27C. The tank is filled such that there are no voids (air pockets) within. The initial pressure within the...
-
Suppose the force on an object varies with time as shown in Figure P7.19. Estimate the impulse imparted to the object. Assume the motion is one dimensional. Figure P7.19 ? F (N)_ 3000 2000 1000 -t...
-
An astronaut is working in distant space (outside his spaceship and far from any planets or stars) and floating freely when he accidently throws a wrench. (a) Is the astronauts momentum conserveda...
-
Uppercut! A boxer hits an opponent on the chin and imparts an impulse of 500 N s. Estimate the magnitude of the average force. You will need to estimate the collision time, that is, the time two...
-
Q1) The equity of Washington Ltd at 1 July 2020 consisted of: Share capital 500 000 A ordinary shares fully paid $1 500 000 400 000 B ordinary shares issued for $2 and paid to $1.50 600 000 General...
-
out The following information relates to Questions 1 to 2. The management accountant of a furniture manufacturer is developing a standard for the labour cost of one massage chair. When operating at...
-
Exercise 10-8 Utilization of a constrained Resource [LO10-5, L010-6] Barlow Company manufactures three products: A, B, and C. The selling price, variable costs, and contribution margin for one unit...

Study smarter with the SolutionInn App


