From the data presented in Figure 12.11, determine which has the more positive enthalpy of solution: NaCl
Question:
From the data presented in Figure 12.11, determine which has the more positive enthalpy of solution: NaCl or KNO3. Explain.
Figure 12.11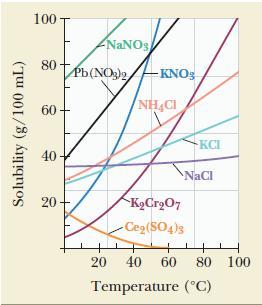
Transcribed Image Text:
Solubility (g/100 mL) 100 80 60 40 20 NaNOs Pb (NO3)2KNO3 NH4CI KCrO7 -Ce(SO4)3 KCI NaCl 20 40 60 80 100 Temperature (C)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (2 reviews)
The enthalpy of solution Delta Htextsol can be indirectly inferred from a solubility versus temperat...View the full answer

Answered By

Dorcas Juliet
I am a proficient tutor and writer with over 4 years experience, I can deliver A+ works in all fields related to business and economics subject. Kindly hire me for excellent papers
4.70+
10+ Reviews
51+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
From the data presented in Figure 12.11, determine which has the more positive enthalpy of solution: NaCl or NH 4 Cl. Explain. Figure 12.11 Solubility (g/100 mL) 100 80 60 40 20 NaNO3 Pb(NO3)2KNO3...
-
Reconsider the Special Products Company problem presented in Section 1.2. Although the company is well qualified to do most of the work in producing the iWatch, it currently lacks much expertise in...
-
The data presented in Table suggest that the corporate form suffered a tax disadvantage relative to the partnership form from 1987 to 1992. List and explain the factors that caused this outcome. Why...
-
Lopez Company uses a job order cost accounting system that charges overhead to jobs on the basis of direct material cost. At year-end, the Goods in Process Inventory account shows the following. 1....
-
An abc-sequence balanced three-phase wye-connected source supplies power to a balanced wye-connected load. The line impedance per phase is 1 + j5, and the load impedance per phase is 25 + j25. If the...
-
What should the project manager have done about the challenges facing this project?
-
As the LM curve gets steeper, the crowding-out effect of fiscal policy worsens. Why?
-
Brads Bicycle Shop sells 21-speed bicycles. For purposes of a cost- volume-profit analysis, t he shop owner has divided sales into two categories, as follows: Seventy percent of the shops sales are...
-
Differentiate between operating activities, investing activities, and financing activities on the Statement of Cash Flows.
-
At 22 C and 1.0 atm, the enthalpy of solution of nitrogen in water is -11.0 kJ/mol, and its solubility is 6.68 10 -4 m. State whether the solubility of nitrogen is greater or less than 6.68 104 m...
-
The solubilities of most gases in water decrease as the temperature increases. Are the enthalpies of solution for such gases negative or positive? Explain your answer.
-
The text discussion of RRA is in terms of the relevance and reliability of the asset valuation of oil and gas reserves. RRA can also be evaluated in terms of the criteria for revenue recognition....
-
February 12, 2009 marked the 200th anniversary of Charles Darwin's birth. To celebrate, Gallup, a national polling organization, surveyed 1,018 randomly selected American adults about their education...
-
Question 1 (30 points) A 3D infinite quantum well is a very simple model for an atom. Suppose that two cubic 3D infinite quantum wells, with cube dimension L, are joined to form one parallelepiped...
-
Give an algorithm for printing all the ancestors of a node in a Binary tree. For the tree below, for 7 the ancestors are 137. root 4 2 3 5 6 7
-
Consider k measurements that are corrupted by zero-mean Gaussian noise with s.d. , i.e., z=x+wi, i = 1,..., k (6) where x is a constant and w; ~N(0,0). The goal is to estimate the mean x and the...
-
1. For an inviscid flow, the momentum equation for a Newtonian flow can be written as: -(puu;)+ Jxi (pu)+ at 2x j where p is the density and is the pressure. = = 0 (1) (a) In order to characterise a...
-
What is the difference between the isoelectric pH and the isoionic pH of a protein with many different acidic and basic substituents?
-
Velshi Printers has contracts to complete weekly supplements required by fortysix customers. For the year 2018, manufacturing overhead cost estimates total $600,000 for an annual production capacity...
-
Determine the force in each member of the roof russ. State if the members are in tension or compression. 8 kN 4 kN 4 kN 4 kN 4 kN - 4 kN - | 3.5 m 4 kN 6 @ 4 m = 24 m
-
Determine the force in each member of the roof truss. State if the members are in tension or compression. Assume all members are pin connected. 3 m E- |B |C 10 kN 10 kN 10 kN -4 m-4m-4 m-4 m-
-
Determine the force in each member of the truss. State if the members are in tension or compression. 30 30 45 45 30 30 - 2 m 2 kN
-
Estimate the intrinsic value of the stock company ABC. Dividends were just paid at $8 per share and are expected to grow by 5%. You require 20% on this stock given its volatile characteristics. If...
-
Crane, Inc., a resort management company, is refurbishing one of its hotels at a cost of $6,794,207. Management expects that this will lead to additional cash flows of $1,560,000 for the next six...
-
Match each of the following transactions with the applicable internal control principle that is being violated

Study smarter with the SolutionInn App


