Give the notation (1s, 2s, 2p, and so on) for each of the following subshells. If the
Question:
Give the notation (1s, 2s, 2p, and so on) for each of the following subshells. If the combination is not allowed, state why.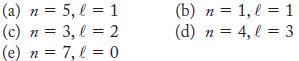
Transcribed Image Text:
(a) n = 5, l = 1 (c) n = 3, l = 2 (e) n = 7, l = 0 (b) n = 1, l=1 (d) n = 4, l = 3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
The notation for subshells follows the format nl where n is the principal ...View the full answer

Answered By

Joseph Njoroge
I am a professional tutor with more than six years of experience. I have helped thousands of students to achieve their academic goals. My primary objectives as a tutor is to ensure that students do not have problems while tackling their academic problems.
4.90+
10+ Reviews
27+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Shown below is a qualitative diagram of the atomic orbital energies for an Na atom. The number of orbitals in each subshell is not shown. (a) Are all of the subshells for n = 1, n = 2, and n = 3...
-
Determine whether each of the following electron configurations represents the ground state or an excited state of the atom given. (a) C (c) Be 1s 2s 1s 2s 2p 2p (b) N 1s 2s (d) ON N 1s 2s 2p 2p
-
A cam is to provide follower motion as follows: The follower rises, with constant velocity, 4 inches in 2 seconds. The follower then dwells for 3 seconds. The follower then falls, 3 inches, with...
-
On 1 June 2019, Manchester United Ltd bought 48 million ordinary shares in Chelsea FC Ltd paying GHS 280 million cash. The summarised statement of financial position for the two entities as at 31...
-
A 4-kg mass is supported by a steel wire of diameter 0.6 mm and length 1.2 m. How much will this wire stretch under this load?
-
Consider the hemoglobin data in Exercise 8-73. Find the following: (a) An interval that contains 95% of the hemoglobin values with 90% confidence. (b) An interval that contains 99% of the hemoglobin...
-
Mongolian desert ants. Refer to the Journal of Biogeography (Dec. 2003) study of ants in Mongolia (central Asia), presented in Exercise 2.68 (p. 91). Recall that botanists placed seed baits at 5...
-
You are the manager of the examination engagement of the financial projection of Honeys Health Foods as of December 31, 2013, and for the year then ended. The audit senior, Currie, has prepared the...
-
Assume an American firm (XYZ) is expected to pay 10m GBP () in one years time. If XYZ hedges its foreign exchange exposure using options, what will be its total dollar payment in one years time,...
-
Understanding the relationship between business and society and the ways in which business and society are part of an interactive system.
-
Give the notation (1s, 2s, 2p, and so on) for each of the following subshells. If the combination is not allowed, state why. l=1 (a) n = 6, (c) n = 5, l = 2 (e) n = 2, l = 3 (b) n = 3,l = 0 (d) n =...
-
Give the values of the n and quantum numbers for the subshells identified by the following designations. (a) 3p (c) 7s (e) 2s (b) 5d (d) 4f 69
-
Show the steps of the CPU fetchexecute cycle for the remaining instructions in the Little Man instruction set.
-
1 . Journalize the following transactions: ( a ) Issued 1 , 0 0 0 shares of $ 1 0 par common stock at $ 5 9 for cash. ( b ) Issued 1 , 4 0 0 shares of $ 1 0 par common stock in exchange for equipment...
-
Using alpha .05, determine if moving to a larger enclosure decreased tiger anxiety levels. You should first calculate the difference (After - Before) Tiger Before Anthony 45 45 Banthony 56 After 38...
-
Cyclohexane (C 6 H 12 ) is produced by mixing Benzene and hydrogen. A process including a reactor, separator, and recycle stream is used to produce Cyclohexane. The fresh feed contains 260L/min C 6 H...
-
Suppose the city is undergoing severe ination. Specifically, both goods prices have risen by 10%. What percentage of a raise in the wage rate should Alex request from her boss, for her to maintain...
-
1. An iron cube of mass 0.55 kg is raised to a temperature of 100C by being placed in boiling water for 5 minutes. It is then removed and transferred immediately to an aluminium calorimeter filled...
-
A 10-cm length of platinum wire 0.4 mm in diameter is placed horizontally in a container of water at 38oC and is electrically heated so that the surface temperature is maintained at 93oC. Calculate...
-
What is a manufacturing system?
-
Some symmetry operations can be carried out physically using a ball-and-stick model of a molecule without disassembly and reassembly and others can only be imagined. Give two examples of each...
-
Predict the number of chemically shifted 1 H peaks and the multiplet splitting of each peak that you would observe for 1,1,1,2-tetrachloroethane. Justify your answer. I H1 --c-CI CI | Cl
-
Predict the number of chemically shifted 1 H peaks and the multiplet splitting of each peak that you would observe for 1, 1, 2, 2-tetrachloroethane assuming that there is no rotation of the two...
-
Ray Company provided the following excerpts from its Production Department's flexible budget performance report. Required: Complete the Production Department's Flexible Budget Performance Report....
-
Problem 1 5 - 5 ( Algo ) Lessee; operating lease; advance payment; leasehold improvement [ L 0 1 5 - 4 ] On January 1 , 2 0 2 4 , Winn Heat Transfer leased office space under a three - year operating...
-
Zafra and Stephanie formed an equal profit- sharing O&S Partnership during the current year, with Zafra contributing $100,000 in cash and Stephanie contributing land (basis of $60,000, fair market...

Study smarter with the SolutionInn App


