How many sigma bonds and how many pi bonds are there in each of the following molecules?
Question:
How many sigma bonds and how many pi bonds are there in each of the following molecules?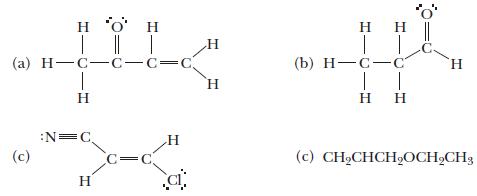
Transcribed Image Text:
| || | (a) H=C=C=C=C | H (c) :N=C H c=c H ic H H (b) H-C-C H T H (c) CHCHCHOCHCH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (2 reviews)
The image depicts four different molecular structures labeled a b c and another c which I believe should have been labeled as d I will provide the num...View the full answer

Answered By

Patrick Busaka
I am a result oriented and motivated person with passion for challenges because they provide me an opportunity to grow professionally.
5.00+
38+ Reviews
58+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
How many carbon-carbon sigma bonds are present in each of the following molecules? (a) 2-butyne. (b) Anthracene (c) 2,3-dimethylpentane
-
How many carbon-carbon sigma bonds are present in each of the following molecules? (a) Benzene, (b) Cyclobutane. (c) 3-ethyl-2-methylpentane
-
b) A firm produces two types of sugar, A and B at a constant average cost of RM 2 and RM3 per kilogram, respectively. The quantities, q and qg (in kilogram) of A and B that can be sold each week are...
-
Days Sales in Receivables a company has net income of $195,000, a profit margin of 9.40 percent, and an accounts receivable balance of $106,851. Assuming 75 percent of sales are on credit, what are...
-
On January 1, 2012, Vaness Corporation was granted a charter authorizing the following capital stock: common stock , $5 par, 200,000 shares; preferred stock, $10 par, 7%, 50,000 shares. Record the...
-
Exit poll candidates and voters. In an exit poll, 45% of voters said that the main issue affecting their choice of candidates was the economy, 35% said national security, and the remaining 20% were...
-
Westan Corporation uses a predetermined overhead rate of $23.10 per direct labor-hour. This predetermined rate was based on 12,000 estimated direct labor-hours and $277,200 of estimated total...
-
on the PV Versus Diesel: A Cost-Benefit Analysis in the Nexus of Food and Fuel problem. Using the case study, provide baseline values for the following. Explain your reasoning behind these responses,...
-
Sketch the bonds in H 2 CNH, label the type of orbital from which each bond forms, and indicate whether the bond is a or a bond.
-
Give the hybridization of each central atom in the following molecules. (a) Cyclohexene (b) Phosgene, Cl 2 CO (c) Glycine, H 2 NC(1)H 2 C(2)OOH H C. H-C || H-C, C C- - C-H H
-
Present value involves converting dollars received or paid in the future to their value in todays dollars. Use your knowledge of present value and the time value of money to analyze the following...
-
Convert the following information into: a) a semantic net b) a frame-based representation A Ford is a type of car. Bob owns two cars. Bob parks his car at home.His house is in California, which is a...
-
Visit www.pearsonglobaleditions.com/malhotra to read the video case and view the accompanying video. Marriott: Marketing Research Leads to Expanded Offerings highlights Marriotts success in using...
-
The water level in a tank is \(20 \mathrm{~m}\) above the ground. A hose is connected to the bottom of the tank, and the nozzle at the end of the hose is pointed straight up. The tank cover is...
-
A simple experiment has long been used to demonstrate how negative pressure prevents water from being spilled out of an inverted glass. A glass that is fully filled by water and covered with a thin...
-
A golf ball is hit on a level fairway. When it lands, its velocity vector has rotated through an angle of 90. What was the launch angle of the golf ball? Pyo By Dyz =0 Uso Range R x max dya
-
A standard solution containing 6.3 10-8 M iodoacetone and 2.0 10-7 M p dichlorobenzene (an internal standard) gave peak areas of 395 and 787, respectively, in a gas chromatogram. A 3.00-mL unknown...
-
Stephen Schor, an accountant in New York City, advised his client, Andre Romanelli, Inc., to open an account at J. P. Morgan Chase Bank, N.A., to obtain a favorable interest rate on a line of credit....
-
The 60-mm-diameter shaft rotates at 300 rev/min. This motion is caused by the unequal belt tensions on the pulley of 800 N and 450 N. Determine the power transmitted and the maximum shear stress...
-
Draw the shear and moment diagrams for the shaft and determine the shear and moment throughout the shaft as a function of x for 0 ¤ x < 3 ft, 3 ft < x < 5 ft, and 5 ft < x < 6 ft. The bearings...
-
Draw the shear and moment diagrams for the beam, and determine the shear and moment in the beam as functions of x for 0 ¤ x < 4 ft, 4 ft < x < 10 ft, and 10 ft < x < 14 ft. 250 Ib 250 lb 150...
-
Product Weight Sales Additional Processing Costs P 300,000 lbs. $ 245,000 $ 200,000 Q 100,000 lbs. 30,000 -0- R 100,000 lbs. 175,000 100,000 If joint costs are allocated based on relative weight of...
-
The projected benefit obligation was $380 million at the beginning of the year. Service cost for the year was $21 million. At the end of the year, pension benefits paid by the trustee were $17...
-
CVP Modeling project The purpose of this project is to give you experience creating a multiproduct profitability analysis that can be used to determine the effects of changing business conditions on...

Study smarter with the SolutionInn App


