Give the hybridization of each central atom in the following molecules. (a) Cyclohexene (b) Phosgene, Cl 2
Question:
Give the hybridization of each central atom in the following molecules.
(a) Cyclohexene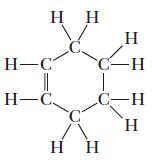
(b) Phosgene, Cl2CO
(c) Glycine, H2NC(1)H2C(2)OOH
Transcribed Image Text:
H C. H-C || H-C, C C- - C-H H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a The double bonded at...View the full answer

Answered By

Sheikh Muhammad Ibrahim
During the course of my study, I have worked as a private tutor. I have taught Maths and Physics to O'Level and A'Level students, as well as I have also taught basic engineering courses to my juniors in the university. Engineering intrigues me alot because it a world full of ideas. I have passionately taught students and this made me learn alot. Teaching algebra and basic calculus, from the very basics of it made me very patient. Therefore, I know many tricks to make your work easier for you. I believe that every student has a potential to work himself. I am just here to polish your skills. I am a bright student in my university. My juniors are always happy from me because I help in their assignments and they are never late.
4.90+
14+ Reviews
24+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Give the hybridization of each central atom in the following molecules. (a) CO 2 (b) H 3 CCCH (c) H 3 CC(O)H, which has the Lewis structure H :O: H-C-C-H H
-
Give the hybridization of the central atom of each of the following species, and tell whether the bond arrangement around it is linear, trigonal planar, or tetrahedral: a. NH3 b. BH3 c. -CH3 d. CH3...
-
Indicate the hybridization on each central atom in the molecules with the following Lewis structures. (a) H :C: H-C-0-N-CI: 1 H (b) H T H-C=C-P-H
-
The given graph is a transformation of one of the six basic functions. Find an equation for the given graph. 10 'y -1 -2 -3 -5 -6 -7 -8 -9 -10 -15 -13 -9 -8 -7 -6 -5 4 3 -2 -1 0 1 2 3 45 -11
-
Using the Du Pont Identity Y3K, Inc., has sales of $4,800, total assets of $2,685, and a debtequity ratio of 1.20. If its return on equity is 16 percent, what is its net income?
-
A computer virus destroyed important financial information pertaining to Paseo Company's stockholders' equity section. Your expertise is needed to compute the missing account balances. The only...
-
Simulate the experiment described in Exercise 3.7 using any five identically shaped objects, two of which are one color and three another color. Mix the objects, draw two, record the results, and...
-
As a prospective owner of a club known as the Red Rose, you are interested in determining the volume of sales dollars necessary for the coming year to reach the break-even point. You have decided to...
-
A bank has total interest income of $67 million and total noninterest income of $14 million. This bank has total interest expenses of $35 million and total noninterest expenses (excluding PLL) of $28...
-
How many sigma bonds and how many pi bonds are there in each of the following molecules? | || | (a) H=C=C=C=C | H (c) :N=C H c=c H ic H H (b) H-C-C H T H (c) CHCHCHOCHCH3
-
Three resonance structures can be written for N 3 . Indicate the hybridization on the central atom for each resonance form.
-
What action should be taken when unacceptable error is found in tracking a forecast?
-
Repeat Prob. 10-18 for signed-magnitude binary numbers. Prob. 10-18 Derive an algorithm in flowchart form for the comparison of two signed binary numbers when negative numbers are in signed-2's...
-
Tideview Home Health Care, Inc., has a bond issue outstanding with eight years remaining to maturity, a coupon rate of 10 percent with interest paid annually, and a par value of $1,000. The current...
-
Captain Billy Whirlywhirl Hamburgers issued 7%, 10-year bonds payable at 70 on December 31, 2010. At December 31, 2012, Captain Billy reported the bonds payable as follows: Captain Billy Whirlywhirl...
-
Two scenarios about the future of the global economy in 2050 have emerged. Known as continued globalization, the first scenario is a (relatively) rosy one. Spearheaded by Goldman Sachs, whose...
-
Visit www.pearsonglobaleditions.com/malhotra to read the video case and view the accompanying video. Nike: Associating Athletes, Performance, and the Brand highlights Nike's use of marketing research...
-
(a) When a solution containing 234 mg of pentanol (FM 88.15) and 237 mg of 2,3-dimethyl 2-butanol (FM 102.17) in 10.0 mL was separated, the relative peak areas were pentanol:2,3 dimethyl-2-butanol...
-
Aztec Furnishings makes hand-crafted furniture for sale in its retail stores. The furniture maker has recently installed a new assembly process, including a new sander and polisher. With this new...
-
Draw the shear and moment diagrams for the beam, and determine the shear and moment throughout the beam as functions of x for 0 x 6 ft and 6 ft x 10 ft.
-
Express the shear and moment in terms of x for 0 < x < 3 m and 3 m < x < 4.5 m, and then draw the shear and moment diagrams for the simply supported beam. 300 N/m 1.5 m
-
Express the internal shear and moment in the cantilevered beam as a function of x and then draw the shear and moment diagrams. 300 Ib 200 lb/ft 6 ft
-
A government bond matures in 30 years, makes semi-annual coupon payments of 6.0% ($120 per year) and offers a yield of 3.7% annually compounded. Assume face value is $1,000. Three years later the...
-
Your objective is: 1. Carry out a life insurance needs analysis, for each one of them (show your calculations) [30 Marks] 2. Refer to the case and the insurance plan quotes. Would you recommend...
-
TufStuff, Incorporated, sells a wide range of drums, bins, boxes, and other containers that are used in the chemical industry. One of the company s products is a heavy - duty corrosion - resistant...

Study smarter with the SolutionInn App


