Indicate the hybridization on each central atom in the molecules with the following Lewis structures. (a) H
Question:
Indicate the hybridization on each central atom in the molecules with the following Lewis structures.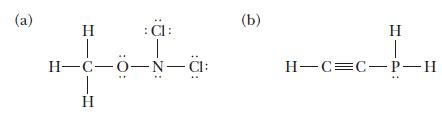
Transcribed Image Text:
(a) H :C: H-C-0-N-CI: 1 H (b) H T H-C=C-P-H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
a C sp ...View the full answer

Answered By

Asim farooq
I have done MS finance and expertise in the field of Accounting, finance, cost accounting, security analysis and portfolio management and management, MS office is at my fingertips, I want my client to take advantage of my practical knowledge. I have been mentoring my client on a freelancer website from last two years, Currently I am working in Telecom company as a financial analyst and before that working as an accountant with Pepsi for one year. I also join a nonprofit organization as a finance assistant to my job duties are making payment to client after tax calculation, I have started my professional career from teaching I was teaching to a master's level student for two years in the evening.
My Expert Service
Financial accounting, Financial management, Cost accounting, Human resource management, Business communication and report writing. Financial accounting : • Journal entries • Financial statements including balance sheet, Profit & Loss account, Cash flow statement • Adjustment entries • Ratio analysis • Accounting concepts • Single entry accounting • Double entry accounting • Bills of exchange • Bank reconciliation statements Cost accounting : • Budgeting • Job order costing • Process costing • Cost of goods sold Financial management : • Capital budgeting • Net Present Value (NPV) • Internal Rate of Return (IRR) • Payback period • Discounted cash flows • Financial analysis • Capital assets pricing model • Simple interest, Compound interest & annuities
4.40+
65+ Reviews
86+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Three resonance structures can be written for N 3 . Indicate the hybridization on the central atom for each resonance form.
-
Two resonance structures can be written for NO 2 . Indicate the hybridization on the central atom for each resonance form.
-
Give the hybridization of each central atom in the following molecules. (a) CO 2 (b) H 3 CCCH (c) H 3 CC(O)H, which has the Lewis structure H :O: H-C-C-H H
-
a) If the block is at rest (and the only forces acting on the block are the force due to gravity and the normal force from the table), what is the magnitude of the force due to friction? b) Suppose...
-
Du Pont Identity Construct the Du Pont identity for Smolira Golf Corp.
-
In the lecture, Professor Murayama talked about how we can use cosmic ray muons to map otherwise invisible things. A particularly novel example he discussed was Luis Alvarez looking for a hidden...
-
Self-employed in Finland. Based on a random sample drawn twice a year from the Statistics Finland population database, the Labour Force Survey collects statistical data on the participation in work,...
-
Gordon & Moore, CPAs, were the auditors of Fox & Company, a brokerage firm. Gordon & Moore examined and reported on the financial statements of Fox, which were filed with the Securities and Exchange...
-
30) Merchandise inventory at the end of the year is overstated. Which of the following statements correctly states the effect of the error? Onet income is understated Downer's equity is overstated...
-
Nitrous acid has the skeleton structure HONO. What are the hybrid orbitals on the nitrogen atom and the central oxygen atom?
-
Indicate the hybridization on each central atom in the molecules with the following Lewis structures. (a) :: T H=C=C=C-H | (b) T H=C=C=N: H
-
For the clutch of Prob. 627, the external load P is cycled between 20 kN and 80 kN. Assuming that the shaft is rotating synchronous with the external load cycle, estimate the number of cycles to...
-
The adjusted trial balance section of Menlo Company's worksheet shows a \(\$ 1,500\) debit balance in utility expense. At the end of the accounting period the accounting manager accrues an additional...
-
Identify each of the 10 amount columns of the worksheet and indicate to which column the adjusted balance of the following accounts would be extended: a. Accounts Receivable b. Accumulated...
-
Using the data from Table 3.3, show the effect on world output if each country moved toward specialization in the production of its comparative-disadvantage good. TABLE 3.3 Comparative Advantage as a...
-
The Professional Winner was RJ Andrews from Info We Trust, for the video Are Gazelles Endangered? (a) Watch this video. What data are this video conveying? (b) You can interact with the data and...
-
(a) Draw a simplified ray diagram showing the three principal rays for an object located outside the focal length of a converging lens. (b) Is the image real or virtual? (c) Is it upright or...
-
Equation 23-3 gives the mass of analyte extracted into a solid-phase microextraction fiber as a function of the partition coefficient between the fiber coating and the solution. (a) A commercial...
-
Comptech Ltd is a manufacturer of optical equipment. In September 2019, Ed Thompson the Chief Research Officer, attended a conference in Switzerland that focused on optical developments for the 21st...
-
The shaft is used to transmit 30 hp while turning at 600 rpm. Determine the maximum shear stress in the shaft. The segments are connected together using a fillet weld having a radius of 0.18 in. 2,5...
-
The built-up shaft is designed to rotate at 450 rpm. If the radius of the fillet weld connecting the shafts is r = 13.2 mm, and the allowable shear stress for the material is Ï allow = 150 MPa,...
-
The built-up shaft is to be designed to rotate at 450 rpm while transmitting 230 kW of power. Is this possible? The allowable shear stress is Ï allow = 150 MPa. 100 mm 60 mm
-
A zero-coupon bond bears a higher interest rate risk than a coupon-paying bond, given other bond characteristics are equal. True False
-
Company management decided to restructure its balance sheet. Current long term debt of 8 mio euros will be increased to 20 mio euros. Interest for the debt is 4%. Borrowed 12 mio euros will used to...
-
Susan loans 10000 to Jim. Jim repays the loan with yearly instalments at the end of each year. Interest is expected to be 5% the first five years, and 10% the last five years. Calculate the...

Study smarter with the SolutionInn App


