If a volume of 32.45 mL HCl is used to completely neutralize 2.050 g Na 2 CO
Question:
If a volume of 32.45 mL HCl is used to completely neutralize 2.050 g Na2CO3 according to the following equation, what is the molarity of the HCl?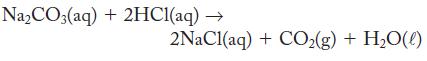
Transcribed Image Text:
Na₂CO3(aq) + 2HC1(aq) → 2NaCl(aq) + CO₂(g) + H₂O(0)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
To determine the molarity of the HCl solution we need to use the volume and mass data pro...View the full answer

Answered By

Ishrat Khan
Previously, I have worked as an accounting scholar at acemyhomework, and have been tutoring busines students in various subjects, mostly accounting. More specifically I'm very knowledgeable in accounting subjects for college and university level. I have done master in commerce specialising in accounting and finance as well as other business subjects.
5.00+
140+ Reviews
437+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
1. What mass of H2 should be produced by the reaction of Al with 75.0 mL of 2.95M HCl? 2Al(s) + 6HCl(aq) 2AlCl3(aq) + 3H2 (g). ln the lab, 0.15g H2 was collected. What is the % yield of the...
-
If 25.30 mL of 0.277 M HCl is used to titrate 10.0 mL of aqueous ammonia to a methyl red endpoint, what is the molarity of the ammonia? When we write aqueous ammonia as NH 4 OH, the balanced equation...
-
The volume of a sample of pure HCl gas was 189 mL at 25C and 108 mmHg. It was completely dissolved in about 60 mL of water and titrated with an NaOH solution; 15.7 mL of the NaOH solution were...
-
The adjusted trial balance for Ray Corporation at July 31, 2017, the corporation's fiscal year end, contained the following: Of the lease liability amount, $16,250 is due within the next year. Total...
-
We consider the random process St, which plays a fundamental role in BIack-Scholes analyses: St = S0e[1+Wt] Where Wt is a Wiener process with W0 = 0, is a trend factor, and (Wt Ws) N(0, (I s)),...
-
Question 8 (3 points) Which of the following is a "preventative control" (versus a detective control)? Taking periodic inventory. Month-end bank reconciliations. Periodically checking customer...
-
What advice can you offer Nick to help him with his procrastination? LO.1
-
Par Company acquires 100% of the common stock of Sub Company for an agreed-upon price of $900,000. The book value of the net assets is $700,000, which includes $50,000 of subsidiary cash equivalents....
-
LCD Industries purchased a supply of electronic components from Entel Corporation on November 1, 2021. In payment for the $24.7 million purchase, LCD issued a 1-year installment note to be paid in...
-
The comparative balance sheet of Olson-Jones Industries Inc. for December 31, 20Y2 and 20Y1, is as follows: The following additional information is taken from the records: A. Land was sold for $120....
-
What is the molar concentration of a solution of HNO 3 if 50.00 mL react completely with 22.40 mL of a 0.0229 M solution of Sr(OH) 2 ?
-
Write the overall equation (including the physical states), the complete ionic equation, and the net ionic equation for the reaction of aqueous solutions of sodium hydroxide and magnesium chloride....
-
Opportunity costs are: a. explicit costs b. the value of a resource in its current use c. implicit costs d. the value of a resource in its previous use
-
Matthew Kennedy of Urbana, Ohio, is single and has been working as an admissions counselor at a university for five years. Matthew owns a home valued at $250,000 on which he owes $135,000. He has a...
-
Question: A group of employees of Unique Services will be surveyed about a new pension plan. In-depth interviews with each employee selected in the sample will be conducted. The employees are...
-
On January 1, 2020, the following accounts appeared in the general ledger of Ace's Repair Shop: Cash P10,500 Accounts receivable 8.400 Furniture 12,600 Repair Equipment 54,000 Accounts Payable 22,000...
-
Your maths problem x+3x-3 Find solutions on the web Q +1 XII
-
5. Data for the payroll for the Dos Company for the month of April are shown below: Total gross earnings Social security taxes withheld Phil Health taxes withheld Employees income tax withheld...
-
On January 1, 2018, the date of bond authorization, Paquette Inc. issued 3-year, 12-per cent bonds. Semi-annual interest is payable on June 30 and December 31. Paquette uses the straight-line method...
-
The power company must generate 100 kW in order to supply an industrial load with 94 kW through a transmission line with 0.09 resistance. If the load power factor is 0.83 lagging, find the...
-
Predict the products for each of the following: a. b. c. d. 1) Hg(OAc), 2) NABH, 1) Hg(OAc), e 2) NaBH,
-
Starting with benzene and using any other necessary reagents of your choice, design a synthesis for each of the following compounds. Each compound has a Br and one other substituent that we did not...
-
Benzene was treated with (R)-2-chlorobutane in the presence of aluminum trichloride, and the resulting product mixture was found to be optically inactive. (a) What products are expected, assuming...
-
Each week you must submit an annotated bibliography. Entries of current events relating to the economic concepts and the impact on the company or the industry of your company. You must use acceptable...
-
Fluffy Toys Ltd produces stuffed toys and provided you with the following information for the month ended August 2020 Opening WIP Units 5,393 units Units Started and Completed 24,731 units Closing...
-
Part A Equipment 1,035,328 is incorrect Installation 44,672 is incorrect Anything boxed in red is incorrect sents 043/1 Question 9 View Policies Show Attempt History Current Attempt in Progress...

Study smarter with the SolutionInn App


