In each part, an orbital diagram for an atom is given. Identify the element and whether this
Question:
In each part, an orbital diagram for an atom is given. Identify the element and whether this is the ground state of the atom. For any excited states, show the orbital diagram for the ground state.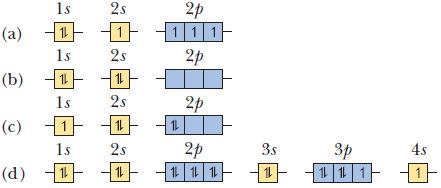
Transcribed Image Text:
1s (a) 11 1s (b) 11 1s |1| 1s 11 (c) (d) 2s 2s 11 2s 11 2s 11 2p 111 2p 12 2p 2p 12 12 12 3s 1 3p 1 1 1 4s -11-
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
The orbital diagrams provided depict the electron configurations of different elements in various st...View the full answer

Answered By

Shristi Singh
A freshman year metallurgy and material science student in India.
4.80+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
In each part, an orbital diagram for an atom is given. Identify the element and whether this is the ground state of the atom. For any excited states, show the orbital diagram for the ground state....
-
Given the valence electron orbital level diagram and the description, identify the element or ion. a. A ground state atom b. An atom in an excited state (assume two electrons occupy the 1s orbital)...
-
Write an orbital diagram for the ground state of the phosphorus atom (Z = 15). Write all orbitals.
-
The condensed balance sheet and income statement data for Cardinal Corporation are presented below. Additional information: 1. The market price of Cardinal common stock was $5.00, $3.50, and $2.30...
-
Each of the objects shown in figure is suspended from the ceiling by a thread attached to the point marked x on the object. Describe the orientation of each suspended object with a diagram. J10 cm...
-
Cloud seeding has been studied for many decades as a weather modification procedure (for an interesting study of this subject, see the article in Techno metrics by Simpson, Alsen, and Eden, A...
-
Do sets of countries differ in their attitudes toward homosexuality? One way to address this question is to examine the WVS variable NEIGHHOMO: the percentage of people who say they would not want...
-
Using the given information, calculate the gross requirements for each of the components when the company plans to build 100 of its Q Models if you have these inventories: 150 units of component T...
-
Please help!! and please show steps I need to understand how to do this! BBP,LLC Quality Advisors is a small accounting firm offering quality audits and advising services to small and mid-sized...
-
Skylar and Walter Black have been married for 25 years. They live at 883 Scrub Brush Street, Apt. 52B, Las Vegas, NV 89125. Skylar is a stay-at-home parent and Walt is a high school teacher. His W-2...
-
Give the maximum number of electrons that may occupy the following shells or subshells. (a) The 3p subshell (b) The 4d subshell (c) The fourth principal shell (d) The third principal shell
-
The speed of sound waves in air is 344 m/s, and the frequency of middle C is 512 Hz. What is the wavelength (in m) of this sound wave?
-
Solve Problem and graph on a real number line. 2(x + 4) > 5x - 4
-
How do you manage global and international teams? What would you do different?
-
Your friend is super excited about the results of their study! They examined whether different parenting styles [A] resulted in differences in anxiety levels among children. The different levels (a)...
-
Test the series for convergence or divergence. n=1 e1/n 78 O convergent O divergent
-
How do employees perceive the organization's vision and mission, and to what extent do these perceptions influence their commitment to the organization ?
-
In the table below which shows class taken and grade achieved, find the probability that a student selected takes Stat or receives a B grade. Round your answer to three decimal places 40 70 70 50 40...
-
A vertical flat plate is maintained at a constant temperature of 120oF and exposed to atmospheric air at 70oF. At a distance of 14 in. from the leading edge of the plate the boundary layer thickness...
-
Determine whether the lines are parallel, perpendicular, or neither. 2x + 3y = -12, 2y - 3x = 8
-
What is the high-T approximation for rotations and vibrations? For which of these two degrees of freedom do you expect this approximation to be generally valid at room temperature?
-
In constructing the vibrational partition function, we found that the definition depended on whether zero-point energy was included in the description of the energy levels. However, the expression...
-
Although the vibrational degrees of freedom are generally not in the high-T limit, is the vibrational partition function evaluated by discrete summation?
-
How do external factors such as changing consumer preferences affect the retail industry?"
-
Production costs that are not attached to units that are sold are reported as: Cost of goods sold Selling expenses Administrative costs Inventory
-
Please show workings :) Oxford Company has limited funds available for investment and must ration the funds among four competing projects. Selected information on the four projects follows: Life of...

Study smarter with the SolutionInn App


