In each part, arrange the subshells in order of increasing energy in a multielectron atom. (a) 5p,
Question:
In each part, arrange the subshells in order of increasing energy in a multielectron atom.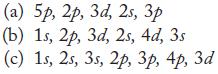
Transcribed Image Text:
(a) 5p, 2p, 3d, 25, 3 (b) 1s, 2p, 3d, 2s, 4d, 3s (c) 1s, 2s, 3s, 2p, 3p, 4p, 3d
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
a 2 s 2p 3p 3d ...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
In each part, arrange the orbitals in order of increasing energy in a multielectron atom. 4s (a) 3px, 2s, Adxy, 35, 4, 3, 45 (b) 1s, 3px, 3dxy 4s, (c) 2s, 4s, 3px, 3dxz, 5s, 3xy
-
Arrange each group of compounds in order of increasing acidity. (a) Phenol, ethanol, acetic acid (b) P-toluenesulfonic acid, acetic acid, chloroacetic acid (c) Benzoic acid, o-nitrobenzoic acid,...
-
Place these types of radiation in order of increasing energy per photon. (a) Green light from a mercury lamp, (b) X-rays from a dental X-ray, (c) Microwaves in a microwave oven, (d) An FM music...
-
As you might imagine, the chart of accounts for a manufacturing firm would be different from that of a service firm. Not surprisingly, service firms differ so much that software now exists for almost...
-
A cylinder of mass M and radius R rolls against a step of height h as shown in Figure. When a horizontal force F is applied to the top of the cylinder, the cylinder remains at rest. (a) What is the...
-
Figure shows a portion of a silver ribbon with z1 = 11.8 mm and y1 = 0.23 mm, carrying a current of 120 A in the +x-direction. The ribbon lies in a uniform magnetic field, in the y-direction, with...
-
Does education also affect ones propensity to believe in various conspiracy theories? Use the ANES Non-Mainstream Views Index as your dependent variable and the four education-level reference-group...
-
On March 31, 2014, Kornet Investments paid $4,480,000 for a tract of land and two buildings on it. The plan was to demolish Building 1 and build a new store (Building 3) in its place. Building 2 was...
-
Ace Service Company issued common stock for proceeds of $ 5 5 8 , 0 0 0 during 2 0 2 5 . The company paid dividends of $ 9 9 , 0 0 0 and issued a long - term note payable for $ 3 7 5 , 0 0 0 in...
-
Your client, Summerford, Inc., has a debt agreement with Valley City Bank that includes a number of restrictions and covenants. Violation of any restriction or covenant results in the entire amount...
-
The absorption spectra of ions have been used to identify the presence of the elements in the atmospheres of the Sun and other stars. (In fact, the element helium was discovered in the spectrum of...
-
For all elements with Z 10, write the electron configuration for (a) Those that have two unpaired electrons. (b) The element with the largest number of unpaired electrons. (c) Those that have only...
-
What do entity-level controls impact?
-
Case study: Sun City - improving operations performance to enhance guest experience 1. Describe how Sun City implements the five operations performance objectives or principles. 2. Using your...
-
What recommendations do you have to increase the likelihood of success? E.g., how would you reduce the likelihood of having to go back to A4? How would you reduce the impact of having to go back to...
-
Problem 4 An electrically heated, square plate (0.4mx 0.4 mx0.005 m) is suspended in air of temperature Too = 20C. Find the electrical power needed to maintain the plate at T=95C if the plate is (a)...
-
Number of units Unit Cost Sales Beginning inventory 800 $50 Purchased 600 $52 Sold 400 $80 Sold 350 $90 Ending inventory 650 In the table below, calculate the dollar value for the period for each of...
-
10. Dr. D went to MGM Springfield casino while the class was taking their midterm exam. He played a Konami machine entitled 88 Fortunes. A slot attendant accidently left the slot manual next to the...
-
A circular air-conditioning duct carries cool air at 5oC and is constructed of 1 percent carbon steel with a thickness of 0.2 mm and an outside diameter of 18 cm. The duct is in a horizontal position...
-
Splitting hairs, if you shine a beam of colored light to a friend above in a high tower, will the color of light your friend receives be the same color you send? Explain.
-
How does one calculate the number of microstates associated with a given configuration?
-
Describe what is meant by the phrase the dominant configuration.
-
What is an occupation number? How is this number used to describe energy distributions?
-
Palisade Creek Co. is a merchandising business that uses the perpetual inventory system. The account balances for Palisade Creek Co. as of May 1, 2019 (unless otherwise indicated), are as follows:...
-
1-When accounting for an acquisition, goodwill is the difference between what two things? 2- What factors should be considered when deciding whether an acquisition should be financed with cash or...
-
What is the main friction Fluidity aims to address? REAL STATE

Study smarter with the SolutionInn App


