In the cubical container below, each dot represents 0.10 of a mole of gas. If the container
Question:
In the cubical container below, each dot represents 0.10 of a mole of gas. If the container volume is 2.3 L and is at 27 °C, calculate the pressure in the container.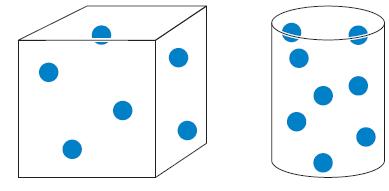
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)

Answered By

Branice Buyengo Ajevi
I have been teaching for the last 5 years which has strengthened my interaction with students of different level.
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Castle Entertainment sells tv/movie memorabilia. The owner (who still wishes to remain anonymous but goes by the name of Richard) has decided that now is right time to once again update his...
-
Explain (with examples if possible) the difference between competitive isomorphism, "Best - fit" or contingency approach to HRM, and the resource based view of the firm.
-
Three brothers, Daniel, David and Derrick have been discussing their respective taxation affairs and how much they dislike paying tax. None of them are Scottish taxpayers. Daniel's income for tax...
-
A constant-volume gas thermometer reads 50 torr at the triple point of water. (a) What will the pressure be when the thermometer measures a temperature of 300 K? (b) What ideal-gas temperature...
-
In Figure P31.30, the bar magnet is moved toward the loop. Is Va - Vb positive, negative, or zero? Explain. Motion toward the loop
-
Demonstrate that the matrix ij = ij is positive semi-definite for all 1 i 1 3
-
Beth died on May 8, 2015. Her executor elected date-of-death valuation. Beths gross estate included, among other properties, the items listed below. What is the estate tax value of each item? a....
-
True 5 pts The amount that the insured pays to purchase insurance protection is face value
-
There is an array A made of N integers. Your task is to choose as many integers from A as possible so that, when they are put in ascending order, all of the differences between all pairs of...
-
Calculate the volume of a gas sample containing 2.35 10 25 water molecules at 0.173 atm of pressure and 229 C.
-
How many N 2 molecules are in a 33.2-L container that is at 1.13 atm of pressure and 122 C?
-
A valve manufacturer has received an inquiry from a company seeking large units for an oil pipeline. Certain types currently in production might be suitable, but they are much smaller than what is...
-
Do you think digital wallets will revolutionize electronic banking and in-store transactions? 2. How do you think digital wallets will affect traditional banks? 3. What are some of the risks of...
-
5.14 Strains are measured on the surface of a brass alloy part as follows: Ex 160010-6 y=1300106, and Yxy = 1500106. Estimate the in-plane stresses x, y, and Txy, and also the strain normal to the...
-
E) prepare preclosing trial balances at december 31,2026. for the debt service fund, considering only the proceeds, expenditures, and transfers resulting from transactions of the capital projects...
-
Explain at least 8 types of Google ads brieflyAnalyze the ad & share your opinion on its performance and suggest changes if required. * add the snapshots, and pictures of examples
-
Categorize each variable as quantitative or qualitative GPA is continuous Number of students is Discrete GPA ( Continuous) and Number of Students ( Discrete) GPA ( Discrete) and the Number of...
-
Suppose that liquid iron is undercooled homogeneous nucleation occurs. Calculate. (a) The critical radius of the nucleus required; and (b) The number of iron atoms in the nucleus. Assume that the...
-
Apply Jacobis method to the given system. Take the zero vector as the initial approximation and work with four-significant-digit accuracy until two successive iterates agree within 0.001 in each...
-
The rotational energy of 7 Li 2 in the J = 5 state is 4.0126 10 22 J. Calculate the bond length of the molecule.
-
A simulated infrared absorption spectrum of a gas-phase organic compound is shown in the following figure. Use the characteristic group frequencies listed in Section 19.5 to decide whether this...
-
An infrared absorption spectrum of an organic compound is shown in the following figure. Use the characteristic group frequencies listed in Section 19.5 to decide whether this compound is more likely...
-
An 8%, 30-year semi-annual corporate bond was recently being priced to yield 10%. The Macaulay duration for this bond is 10.2 years. What is the bonds modified duration? How much will the price of...
-
Question 7 of 7 0/14 W PIERDERY Current Attempt in Progress Your answer is incorrect Buffalo Corporation adopted the dollar value LIFO retail inventory method on January 1, 2019. At that time the...
-
Cost of debt with fees . Kenny Enterprises will issue a bond with a par value of $1,000, a maturity of twenty years, and a coupon rate of 9.9% with semiannual payments, and will use an investment...

Study smarter with the SolutionInn App


