A simulated infrared absorption spectrum of a gas-phase organic compound is shown in the following figure. Use
Question:
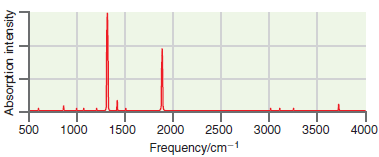
Transcribed Image Text:
2000 500 1000 1500 2500 3000 3500 4000 Frequency/cm-1 Absorption intensity
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (16 reviews)
The peak near 3700 cm 1 is indicative of an OH s...View the full answer

Answered By

Sumit kumar
I am an experienced online essay writer with a thorough understanding of any curriculum.and subject expert at Chegg for mathematics, CS subjects..
4.90+
5+ Reviews
13+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A simulated infrared absorption spectrum of a gas phase organic compound is shown in the following figure. Use the characteristic group frequencies listed in Section 19.5 to decide whether this...
-
An infrared absorption spectrum of an organic compound is shown in the following figure. Use the characteristic group frequencies listed in Section 19.5 to decide whether this compound is more likely...
-
An infrared absorption spectrum of an organic compound is shown in the following figure. Use the characteristic group frequencies listed in Section 19.5 to decide whether this compound is more likely...
-
A credit card contains 16 digits. It also contains a month and year of expiration. Suppose there are one million users of a credit card with unique card numbers. A hacker randomly selects a 16 digit...
-
Consider the function f(x) = cos 1 / x and its graph, shown in the figure below. (a) What is the domain of the function? (b) Identify any symmetry and any asymptotes of the graph. (c) Describe the...
-
In Problems 4043, solve each absolute value inequality. Express your answer using set-builder notation or interval notation. Graph the solution set. 13x +41
-
Understand how aspects of organizational policies and work impact stress experienced by associates. LO5
-
The units of an item available for sale during the year were as follows: There are 50 units of the item in the physical inventory at December 31. The periodic inventory system is used. Determine the...
-
Avia Company soils a product for 500 per unit Variable costs are $50 per unit, and fixed costs are $600 per month. The company expects to sell 600 units in September. The unit contribution margin: O...
-
A store maintains data on customers, products and purchase records in three tables: CUSTOMER, PRODUCT, PURCHASE. The store manager wants to know which product is on its maximum discount for each...
-
The rotational energy of 7 Li 2 in the J = 5 state is 4.0126 10 22 J. Calculate the bond length of the molecule.
-
If the vibrational potential is not harmonic, the force constant is not independent of degree of stretching or compression of a molecule. Using the relation k effective = (d 2 V(x)/dx 2 ), derive an...
-
(a) In relation to property, plant and equipment, define the terms "depreciation", "depreciable amount", "useful life" and "residual value". (b) On 1 July 2019, a company which prepares financial...
-
What volumetric airflow rate (Qa) is required to maintain a G value of 500s-1 in a basin that is 2.75 m deep and provides a liquid detention time of 5 min? Perform the exercise for water temperatures...
-
Read this article, then answer the following questions: 1- Description of Instrument: a. Title of instrument, author(s), publication date 2/18/24, 5:43 PM Abuse Risk Inventory for Women: EBSCOhost...
-
Suppose that there is a magnetic field B(x, y, z) = x2 filling a 3D space. The coordinates are set up as a Cartesian coordinate system with = 2. For all the discussions below, ignore the units. a)...
-
A vertical solid cylinder of uniform cross-sectional area A floats in water. The cylinder is partially submerged. When the cylinder floats at rest, a mark is aligned with the water surface. The...
-
Non-manufacturing fixed cost for year 2011 equal to:$60,780 out of which half are Administrative expenses.Administrative expenses are expected to increase by: 10%The total Variable nonmanufacturing...
-
In Exercises 1138, use the given conditions to write an equation for each line in point-slope form and slope-intercept form. Passing through (-3, 6) and (3, -2)
-
Listed below are several terms and phrases associated with basic assumptions, broad accounting principles, and constraints. Pair each item from List A (by letter) with the item from List B that is...
-
In a metered-dose inhaler (MDI), such as those used for asthma medication, medicine is delivered by a compressed-gas propellant. (The device is similar in concept to a can of spray paint.) When the...
-
A stream of propane at an average temperature T = 566R and absolute pressure P = 6.8 atm flows from a hydrocarbon processing plant to a nearby customers production facility. A technician at the...
-
A 150-liter cylinder of carbon monoxide is stored in a 30.7-m 3 room. The pressure gauge on the tank reads 2500 psi when the tank is delivered. Sixty hours later the gauge reads 2245 psi. The...
-
Imagine you are an Investor in the Stock Market. Identify three companies in the Korean Stock Market (KOSPI) where you would like to invest. Explain your answer
-
Domino is 4 0 years old and is married out of community of property with the exclusion of the accrual system to Dolly ( 3 5 ) . They have one child, Domonique, who is 1 1 years old. Domino resigned...
-
YOU ARE CREATING AN INVESTMENT POLICY STATEMENT FOR JANE DOE General: 60 years old, 3 grown children that are living on their own and supporting themselves. She is in a very low tax rate so we don't...

Study smarter with the SolutionInn App


