Measurements of conductivity of solutions of two acids, A and B, produced the following data. Characterize each
Question:
Measurements of conductivity of solutions of two acids, A and B, produced the following data. Characterize each acid as strong or weak.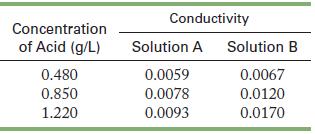
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
A is a ...View the full answer

Answered By

Diksha Bhasin
I have been taking online teaching classes from past 5 years, i.e.2013-2019 for students from classes 1st-10th. I also take online and home tuitions for classes 11th and 12th for subjects – Business Studies and Economics from past 3 years, i.e. from 2016-2019. I am eligible for tutoring Commerce graduates and post graduates. I am a responsible for staying in contact with my students and maintaining a high passing rate.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Two 0.10-mol samples of the hypothetical monoprotic acids HA(aq) and HB(aq) are used to prepare 1.0-L stock solutions of each acid. a. Write the chemical reactions for these acids in water. What are...
-
Classify each acid as strong or weak. If the acid is weak, write an expression for the acid ionization constant (K a ). a. HF b. HCHO c. HSO4 d. HCO3
-
Classify each acid as strong or weak. If the acid is weak, write an expression for the acid ionization constant (K a ). a. HNO3 b. HCI c. HBr d. HSO3
-
Why is it that I got 0 Row with nothing showing up? Is there a flow in my logic? Using AdventureWorks DW2017, list all the Canadian customers who have spent more than $5,000 total. Show Customerkey,...
-
The human brain is especially sensitive to elevated temperatures. The cool blood in the veins leaving the face and neck and returning to the heart may contribute to thermal regulation of the brain by...
-
Roni writes a check for $700 to Sela. Sela indorses the check in blank and transfers it to Titus, who alters the check to read $7,000 and presents it to Union Bank, the drawee, for payment. The bank...
-
E 6-2 Discuss effect of intercompany sale of building Khap Corporation acquired an 80 percent interest in Khun Corporation in 2014. On January 1, 2016, Khap sold a building with a five-year remaining...
-
Some people suggest that the most effective organizations have the strongest cultures. What do we mean by the strength of organizational culture, and what possible problems are there with a strong...
-
prepare an income statement in good form for the X company. assume that there are 350,000 at year-end 75,000 shares were issued in April and 100,000 were issued on sept 30. there were no preferred...
-
Assuming that the conductivity of an acid solution is proportional to the concentration of H 3 O + , sketch plots of conductivity versus concentration for HCl and HF over the 0- to 0.020 M...
-
A solution is prepared by dissolving 0.121 g uric acid, C 5 H 3 N 4 O 3 H (molar mass = 168 g/mol), and diluting to make exactly 10 mL of solution. Each uric acid molecule has only one hydrogen ion...
-
JTextArea components employ line wrap by default. (T / F).
-
You have recently taken over daycare center that was under substandard leadership. Currently, the staff is unmotivated, negative, and often absent from work. You notice that there is minimal...
-
Choose an organization from the industry of your choice to discuss, illustrate, and reflect deliberately on the following: Why is it important to distinguish between "group" and "team "? What kinds...
-
The focus of data governance programs, in some capacity, is enterprise-wide data quality standards and processes. If you were a manager focusing on master data: Would you likely meet enterprise-level...
-
1) Identify and explain each component of the ANOVA model. 2) How is the F ratio obtained? 3) What role does the F ratio play?
-
Make a BCG matrix table and place the following products from Apple: iPhone, iPad, iMac, iPod, Apple TV, Apple Watch, AirPod, and HomePod. Briefly describe why you have placed the products in the...
-
Explain the following observations:
-
Uniform electric field in Figure a uniform electric field is directed out of the page within a circular region of radius R = 3.00 cm. The magnitude of the electric field is given by E = (4.50 x 10-3...
-
Design a multiplier for 16-bit binary integers. Use a design similar to Figures 4-33 and 4-34. (a) Draw the block diagram. Add a counter to the control circuit to count the number of shifts. (b) Draw...
-
The block diagram for an elevator controller for a building with two floors is shown in the following diagram. The inputs FB 1 and FB 2 are floor buttons in the elevator. The inputs CALL 1 and CALL 2...
-
A century ago an entirely new group of elements, the inert gases, was discovered. Is it possible that, in the future, another as yet unknown group of the periodic table might be found?
-
Jen bought 100 shares of ABC stock at $15 a share on July 14, 2017. On August 7, 2018, she noticed that the stock had increased in value to $20 a share and decided to sell her shares. Jen's marginal...
-
Alex. Inci, buys 40 petcent of Steinbart Company on January 1, 2020, for $1.212.000. The equity method of accounting is to be used. Steinbart's net assets on that datewere $2.90 million. Any excess...
-
exercise 4-7 (Algo) Effects of transactions on income statement LO P2

Study smarter with the SolutionInn App


