Phosgene, Cl 2 CO, is an extremely toxic gas that can be prepared by the reaction of
Question:
Phosgene, Cl2CO, is an extremely toxic gas that can be prepared by the reaction of CO with Cl2. Using data from Table 9.4, calculate the approximate enthalpy change for this reaction.
Table 9.4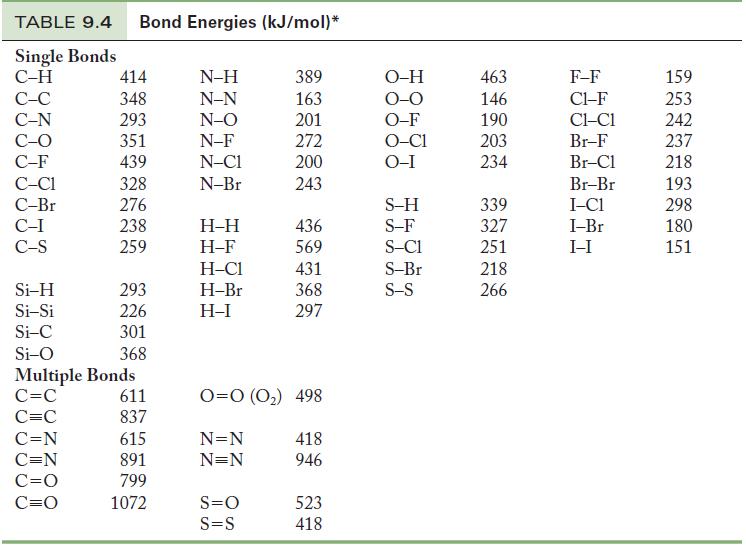
Transcribed Image Text:
TABLE 9.4 Bond Energies (kJ/mol)* Single Bonds C-H C-C C-N C-O C-F C-C1 C-Br C-I C-S Si-H Si-Si Si-C Si-O 414 348 293 351 439 328 276 238 259 C=N C=N C=O C=O 293 226 301 368 Multiple Bonds C=C C=C 611 837 615 891 799 1072 N-H N-N N-O N-F N-C1 N-Br H-H H-F H-C1 H-Br H-I 389 163 201 272 200 243 S=O S=S 436 569 431 368 297 0=0 (0₂) 498 N=N N=N 418 946 523 418 O-H O-O O-F O-C1 O-I S-H S-F S-C1 S-Br S-S 463 146 190 203 234 339 327 251 218 266 F-F C1-F CLC1 Br-F Br-Cl Br-Br I-C1 I-Br I-I 159 253 242 237 218 193 298 180 151
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
To calculate the approximate enthalpy change for the reaction of CO with Cl2 to produce phosgene we ...View the full answer

Answered By

Keziah Thiga
I am a self motivated financial professional knowledgeable in; preparation of financial reports, reconciling and managing accounts, maintaining cash flows, budgets, among other financial reports. I possess strong analytical skills with high attention to detail and accuracy. I am able to act quickly and effectively when dealing with challenging situations. I have the ability to form positive relationships with colleagues and I believe that team work is great key to performance. I always deliver quality, detailed, original (0% plagirism), well-researched and critically analyzed papers.
4.90+
1504+ Reviews
2898+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
You're now starting to set aside money for your future retirement. Here's what you decided you'll do: over the next 30 years - i.e., until you retire - you'll be setting aside the same amount of...
-
a. Which of the following tertiary alcohols cannot be prepared from the reaction of an ester with excess Grignard reagent? b. For those alcohols that can be prepared by the reaction of an ester with...
-
The standard molar enthalpy of formation of diborane, B 2 H 6 (g), cannot be determined directly because the compound cannot be prepared by the reaction of boron and hydrogen. It can be calculated...
-
What important retailing decisions to the target market and retailing marketing mix should be considered when investing in: Dunkin Donuts Little Caesars Pizza Starbucks
-
Compute the percents ionic character of the interatomic bonds for the following compounds: TiO 2 , ZnTe, CsCl, InSb, and MgCl 2 .
-
A lossy material has = 5o, = 2o. If at 5 MHz, the phase constant is 10rad/m, calculate (a) The loss tangent (b) The conductivity of the material (c) The complex permittivity (d) The attenuation...
-
Explicar cmo las organizaciones establecen relaciones duraderas con los clientes y crean valor para estos mediante el marketing.
-
At the beginning of a fiscal year, Alexander Company buys a machine for $ 48,000. The machine has an estimated life of five years and an estimated salvage value of $ 4,000. Required Using the...
-
On January 1, 2015, MNO Company issued $10,000 of 8%, 12 year bonds for $9,632 cash. The bonds are dated January 1, 2018, and pay interest annually each December 31. The market rate of interest on...
-
The molecule nitrosyl chloride, NOCl, has a skeleton structure of O-N-Cl. Two resonance forms can be written; write them both. Use the formal charge stability rules to predict which form is more...
-
Calculate an approximate enthalpy change for the following reaction: HCN(g) + 2H(g) H3CNH(g)
-
Shown, again, in the following table is world population, in billions, for seven selected years from 1950 through 2010. Using a graphing utilitys logistic regression option, we obtain the equation...
-
Find the expected value of buying the extended warranty. The expected value of buying the extended warranty is . A new flat - screen TV comes with a 1 - year warranty wh ich completely covers any...
-
An Olympic runner is coming to visit your school. You have been told that a world class race has two stages. In the first stage, the runner accelerates at a constant rate until he reaches maximum...
-
Question 3(15 marks) An investor holds 100,000 units of a bond whose features per bond are summarized in the following table. She wishes to be hedged against a rise in interest rates. Maturity Coupon...
-
12. In a professional cycling race, competitors (the "peloton") begin the race by riding 45.0 km north, then the road turns west by 37 for the next 50.0 km, before turning due west for the final 35.0...
-
Whateva Ltd uses a flexible budget and standard costs to aid planning and control of its machining manufacturing operations. The costing system for manufacturing has two direct cost categories...
-
Some bacteria use the citric acid cycle intermediate, α-ketoglutarate, plus acetyl-CoA, as the starting point for lysine biosynthesis. The first part of this biosynthetic pathway uses...
-
Following is the current balance sheet for a local partnership of doctors: The following questions represent independent situations: a. E is going to invest enough money in this partnership to...
-
The bars of the truss each have a cross-sectional area of 1.25 in 2 . If the maximum average normal stress in any bar is not to exceed 20 ksi, determine the maximum magnitude P of the loads that can...
-
Determine the size of square bearing plates A² and B² required to support the loading. Take P = 1.5 kip. Dimension the plates to the nearest 1/2 in. The reactions at the supports are...
-
The spring mechanism is used as a shock absorber for a load applied to the drawbar AB. Determine the force in each spring when the 50-kN force is applied. Each spring is originally unstretched and...
-
Mass LLp developed software that helps farmers to plow their fiels in a mannyue sthat precvents erosion and maimizes the effoctiveness of irrigation. Suny dale paid a licesnsing fee of $23000 for a...
-
Average Rate of Return The following data are accumulated by Lone Peak Inc. in evaluating two competing capital investment proposals: 3D Printer Truck Amount of investment $40,000 $50,000 Useful life...
-
4. (10 points) Valuation using Income Approach An appraiser appraises a food court and lounge and provides the following assessment: o O The building consists of 2 floors with the following (6)...

Study smarter with the SolutionInn App


