Predict the geometry, the d orbital energy-level diagram, and the number of unpaired electrons in (a) [Ni(CN)
Question:
Predict the geometry, the d orbital energy-level diagram, and the number of unpaired electrons in
(a) [Ni(CN)4]2- and
(b) [FeBr4]-.
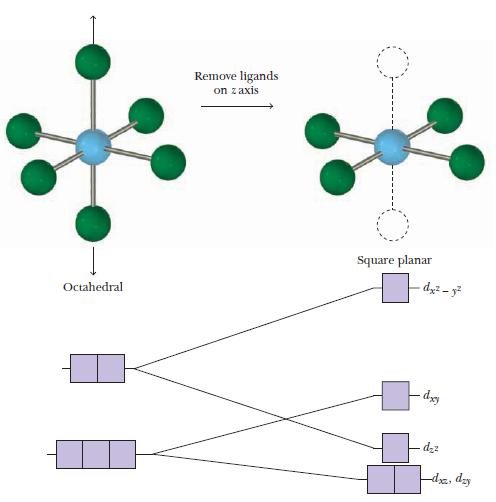
Strategy Based on the d electron configuration of the metal ion, decide whether the four-coordinate complex is tetrahedral or square planar. Use the appropriate diagram from Figure 19.25 or 19.26 to write the orbital energy-level diagram, and determine the number of unpaired electrons.
Figure 19.26
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





