Predict the hybridization at each central atom in the following molecules. (a) | H-C-N: | | (b)
Question:
Predict the hybridization at each central atom in the following molecules.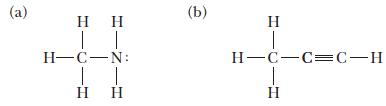
Transcribed Image Text:
(a) | H-C-N: | | (b) H H=C=C=C-H H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
a C and N are ...View the full answer

Answered By

Abigael martinez
I have been a tutor for over 3 years and have had the opportunity to work with students of all ages and backgrounds. I have a strong belief that all students have the ability to learn and succeed if given the right tools and support. I am patient and adaptable, and I take the time to get to know each student's individual learning style in order to best support their needs. I am confident in my ability to help students improve their grades and reach their academic goals.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
In the hydrocarbon (a) What is the hybridization at each carbon atom in the molecule? (b) How many Ï bonds are there in the molecule? (c) How many Ï bonds? (d) Identify all the 120o bond...
-
Use the VSEPR model to predict the bond angles around each central atom in the following Lewis structures (benzene rings are frequently pictured as hexagons, without the letter for the carbon atom at...
-
Indicate the hybridization on each central atom in the molecules with the following Lewis structures. (a) H :C: H-C-0-N-CI: 1 H (b) H T H-C=C-P-H
-
Bill and Mary are married. Mega Corporation employs them both. In 2018, Mary earned $70,000 and Bill earned $135,000, in both cases W-2 wages. How much FICA tax were they required to pay for 2018?
-
Preparing Standardized Financial Statements prepare the 2007 commonbase year balance sheet for Just Dew It. JUST DEW IT CORPORATION 2006 and 2007 Balance Sheets Liabilities and Owners' Equity Assets...
-
Assume that you are using the indirect method of preparing a statement of cash flows. For each of the changes listed, indicate whether it would be added to or subtracted from net income in computing...
-
The sample space for an experiment contains five sample points with probabilities as shown in the table. Find the probability of each of the following events: Sample Points Probabilities 1 .05 2 .20...
-
Selected transactions for Spring Green Lawn Care Company are listed below. 1. Sold common stock for cash to start business. 2. Paid monthly rent. 3. Purchased equipment on account. 4. Billed...
-
15 A deficit in the U.S. current account is offset by a surplus O in the U.S. official reserve assets. in the U.S. trade account. O in the U.S. balance-of-payments. O in the U.S. capital and...
-
Two resonance structures can be written for NO 2 . Indicate the hybridization on the central atom for each resonance form.
-
Predict the hybridization at each central atom in the following molecules. (a) H :S: | || H-C-C-H I H (b) H H 1 H-C-0-C-H H H
-
According to BusinessWeek, environmental groups are making headway on American campuses. In a survey of 570 schools, 130 were found to incorporate chapters of environmental organizations.18 Assume...
-
Ja-San Company was started on January 1,2007, when the owners invested \($160,000\) cash in the business. During 2007, the company earned cash revenues of \($90,000\) and incurred cash expenses of...
-
Write a program using the programming language of your choice to implement the representation you designed for Review Question 3.3. Have your program solve the problem, and have it show on the screen...
-
All the lenses in Figure P33.98 are surrounded by air. Which of the lenses are converging, and which are diverging? Data from Figure P33.98 A B C D E F )(II)
-
Change the Growth and GrowthDriver classes described in the Improved Accuracy and Efficiency. Using a Step-with-Midpoint Algorithm subsection. Run your modified program with these inputs: For your...
-
For the three-element series circuit in Fig. 9-39, (a) Find the current I; (b) Find the voltage across each impedance and construct the voltage phasor diagram which shows that V 1 + V 2 + V 3 = 100 0...
-
State the order of decisions in method development for gas chromatography.
-
In the figure, two loudspeakers, separated by a distance of d1 = 2.63 m, are in phase. Assume the amplitudes of the sound from the speakers are approximately the same at the position of a listener,...
-
Members ABC and BD of the counter chair are rigidly connected at B and the smooth collar at D is allowed to move freely along the vertical post. Draw the shear and moment diagrams for member ABC. -P...
-
A reinforced concrete pier is used to support the stringers for a bridge deck. Draw the shear and moment diagrams for the pier. Assume the columns at A and B exert only vertical reactions on the...
-
Draw the shear and moment diagrams for the beam and determine the shear and moment in the beam as functions of x, where 4 ft < x < 10 ft. 150 Ib/ft 200 lb-ft 200 Ib-ft B- 4 ft ft- 6 ft 4 ft
-
Discuss why it is important for company managers to understand and use social capital knowledge to help build social ties among their skilled knowledge workers so they can build employee loyalty...
-
Kate lives in a house close to a local university, and she traditionally has rented a garage apartment in the back of her property to students for $750 per month. Kate wants to transfer the title to...
-
Pottery Ranch Inc. has been manufacturing its own finials for its curtain rods. The company is currently operating at 100% of capacity, and variable manufacturing overhead is charged to production at...

Study smarter with the SolutionInn App


