Sum the following elementary steps to determine the overall stoichiometry of the hypothetical reaction. Cl C1+ +
Question:
Sum the following elementary steps to determine the overall stoichiometry of the hypothetical reaction.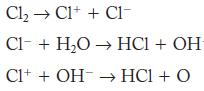
Transcribed Image Text:
Cl C1+ + C1- Cl + HO HC1 + OH C1+ + OH HC1 + O
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
The overall stoichiometry of the reaction is 2Cl 2HO 4HCl O Stepbystep explanatio...View the full answer

Answered By

Aysha Ali
my name is ayesha ali. i have done my matriculation in science topics with a+ . then i got admission in the field of computer science and technology in punjab college, lahore. i have passed my final examination of college with a+ also. after that, i got admission in the biggest university of pakistan which is university of the punjab. i am studying business and information technology in my university. i always stand first in my class. i am very brilliant client. my experts always appreciate my work. my projects are very popular in my university because i always complete my work with extreme devotion. i have a great knowledge about all major science topics. science topics always remain my favorite topics. i am also a home expert. i teach many clients at my home ranging from pre-school level to university level. my clients always show excellent result. i am expert in writing essays, reports, speeches, researches and all type of projects. i also have a vast knowledge about business, marketing, cost accounting and finance. i am also expert in making presentations on powerpoint and microsoft word. if you need any sort of help in any topic, please dont hesitate to consult with me. i will provide you the best work at a very reasonable price. i am quality oriented and i have 5 year experience in the following field.
matriculation in science topics; inter in computer science; bachelors in business and information technology
_embed src=http://www.clocklink.com/clocks/0018-orange.swf?timezone=usa_albany& width=200 height=200 wmode=transparent type=application/x-shockwave-flash_
4.40+
11+ Reviews
14+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Sum the following elementary steps to determine the overall stoichiometry of this hypothetical reaction. NO N + O 03 + 0 202 O + N NO
-
Sum the following elementary steps to determine the overall stoichiometry of the reaction. Cl 2C1 Cl + CO COCI COCI+ CI COC1
-
Sum the following elementary steps to determine the overall stoichiometry of the gas-phase reaction. NO + NO NO3 + NO NO3 + CO NO + CO
-
Two experienced managers at Wilson Boat, Inc. are resisting the introduction of a computerized exponential smoothing system, claiming that their judgmental forecasts are much better than any computer...
-
Find I1 in the network infigure. ww 6 mA 2 mA ww ww
-
What are the four ways that VCs are most commonly organized? How are their deals structured and priced?
-
What advice can you offer Isdell Minnick, and her successor to make the Coca-Cola Co. even more successful? LO.1
-
Use Worksheet 14.1 to help Bill and Shirley Hogan, whod like to retire while theyre still relatively youngin about 20 years. Both have promising careers, and both make good money. As a result, theyre...
-
43. Under the Uniform Commercial Codes rule, a warehouseman. a. Is liable as an insurer b. Will not be liable for the nonreceipt or misdescription of the goods stored even to a good faith purchaser...
-
Write the rate law and the molecularity for each of the following elementary reactions. (a) HCl H + Cl (b) H + Cl HC1 + H (c) 2NO N04
-
A catalyst decreases the activation energy of a particular endothermic reaction by 50 kJ/mol, from 140 to 90 kJ/mol. Assuming that the reaction is endothermic, that the mechanism has only one step,...
-
Is it possible to construct a portfolio of real-world stocks that has a required return equal to the risk-free rate? Explain
-
1.1 Indonesia is it potential as a market for Apple? 2.1 Examination of Apple's entry strategy into the international market? 2.2 Evaluation of the entry mode(s) employed by Apple and their...
-
Dynamic, a global media agency, has recently taken over MediaHype, a local agency in Melbourne, to expand its Australian operations. Jeff Tan, a Chinese national, has been appointed to head the new...
-
Linear optimization models play a crucial role in improving supply chain management efficiency, both in physical and abstract network problems. Three ways they can be applied are through optimizing...
-
When I consider optimizing the portfolio allocation for both my 403(b) and CALSTRS retirement accounts, I find it crucial to employ a well-structured model to ensure that my investments align with my...
-
How can you use your understanding of diversity to develop your relationship-building skills in your healthcare career?,Explain ways in which religion can help or hinder individuals as they build...
-
Isodrin, an isomer of aldrin, is obtained when cyclopentadiene reacts with the hexachloronorbornadiene, shown here. Propose a structure for isodrin. Cl Cl Cl CI sodrin Cl Cl
-
A consultant is beginning work on three projects. The expected profits from these projects are $50,000, $72,000, and $40,000. The associated standard deviations are $10,000, $12,000, and $9,000....
-
Determine the force in member CD of the truss. AE is constant. 4 m 3 m V9 kN l0006 4 m 4 m
-
Determine the force in each member of the pin-connected truss. AE is constant. 3 ft 2 k 2k 3 ft
-
Determine the force in each member of the truss. Assume the members are pin connected at their ends. AE is constant. 20 kN 15 kN 10 kN 'D 2 m 2 m 2 m
-
How do warehouses and distribution centers differ? What is cross-docking and why might a company choose to cross-dock a product? What kinds of products can be delivered electronically? What kinds...
-
Strawberry Inc. has historically been an all-equity firm. The analyst expects EBIT to be $1.5B in perpetuity starting one year from now. The cost of equity for the company is 11.5% and the tax rate...
-
Guzman company received a 60- day, 5 % note for 54,000 dated July 12 from a customer on account. Determine the due date on note. Determine the maturity value of the note and journalize the entry of...

Study smarter with the SolutionInn App


