The chapter introduction introduced the following reaction as one chemical reaction used to launch the space shuttle.
Question:
The chapter introduction introduced the following reaction as one chemical reaction used to launch the space shuttle. Calculate the mass of aluminum required to generate 60,500 kJ energy (enough to launch 1 kg of matter into space). The thermochemical equation is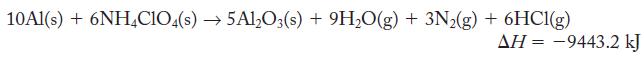
Strategy
The approach is the reverse of that used in Examples 5.1 and 5.2.
Examples 5.1
Calculate the enthalpy change if 5.00 mol N2 (g) reacts with O2 (g) to make NO, a toxic air pollutant and important industrial compound, using the following thermochemical equation:![]()

Examples 5.2
Calculate the enthalpy change observed in the combustion reaction of 1.00 g ethane , using the thermochemical equation.
Thermochemical Equation![]()
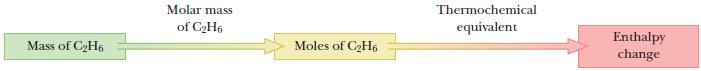
Step by Step Answer:

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball





