Use the accompanying phase diagram to do the following: (a) Label each region of the diagram with
Question:
Use the accompanying phase diagram to do the following:
(a) Label each region of the diagram with the phase that is present.
(b) Identify the phase or phases present at each of the points A, B, C, D, and E.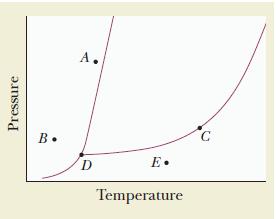
Transcribed Image Text:
Pressure B. A. D E. Temperature
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
a The phases in each region are le...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
The volume of the rectangular solid above is 720. If AF = 15, which of the following is closest to the distance from C to F? 6 8 12 15 18 B F D 6 E
-
Use the accompanying phase diagram to do the following: (a) Label each region of the diagram with the phase that is present. (b) Identify the phase or phases present at each of the points G, H, J,...
-
Use the accompanying phase diagram for sulfur to answer the following questions. (The phase diagram is not to scale.) a. How many triple points are in the phase diagram? b. What phases are in...
-
Your friend recently attended a local mail fraud trial. In your conversation about the case, she described the cross-examination of the expert witness as follows: After his counsels questioning was...
-
A variable-frequency voltage source drives the network in figure. Determine the resonant frequency, Q, BW, and the average power dissipated by the network atresonance. w- 100 n 50 mH 12 cos wf V+ :5 F
-
Ten annual returns are listed in the following table. a. What is the arithmetic average return over the ten-year period? *b. What is the geometric average return over the ten-year period? c. If you...
-
Many psychologists believe that knowledge of a college student's relationships with his or her parents can be useful in predicting the student's future interpersonal relationships both on the job and...
-
Advance inc is trying to determine its cost of debt. The firm has a debt issue outstanding with 12 years to maturity that is quoted at 105% of face value. The issue makes semi-annual payments and has...
-
Tina shorts ABC stock at $65. She closes her position by buying back ABC stock at $71. What is Tina's return on her stock trade? (Use $65 as initial equity invested) A) 8.69% B) 9.23% C) 9.69% D)...
-
The normal melting point of iodine is 113.5 C, and its normal boiling point is 184.3 C. The triple point occurs at 92.3 torr and 113.4 C, and the critical point is 512 C at 112 atm. Sketch the phase...
-
Answer the following questions by using the phase diagram in Exercise 11.44. (a) Sketch the heating curve that is expected when heat is added to the sample at constant pressure, starting at point J....
-
Find the value of the line integral If F is conservative, the integration may be easier on an alternative path.) F(x, y, z) = e z (yi + xj + xyk) (a) r 1 (t) = 4 cos ti + 4 sin tj + 3k, 0 t (b)...
-
The cable supports two cylinders as shown. Cylinders E and F have a mass of 15 kg and 35 kg, respectively. Determine the sag dc and the tension in each segment of the cable. 2 m 2.5 m -2.5m- 2 m dc E...
-
A raft foundation having dimensions of 35 m x 35 m in plan is to be constructed on a deep deposit of sand. Foundation depth and the ground water table are both 5 m below the surface. Unit weight of...
-
Determine the number of 2 X 4 @ 92 5/8" studs needed for the garage in Figures 14.63 and 14.64. The studs are spaced 16 inches on center. Add two studs for each door and corner. Ignore the gable ends...
-
Sketch a cumulative flow diagram that represents the growth and dissipation of a rush hour period at a toll bridge with time-independent capacity. 1) Identify on the diagram: the arrival curve A(t),...
-
Plot the reciprocal lattice for a polycrystalline sample o fa material with a simple tetragonal structure and lattice parameters a = 4.0 A and c = 5.0 A. (Use a two dimensional section through the...
-
State the purpose of an auxiliary complexing agent and give an example of its use.
-
Consider the following cash flows in Table P5.5. (a) Calculate the payback period for each project. (b) Determine whether it is meaningful to calculate a payback period for project D. (c) Assuming...
-
Calculate the density of oxygen gas at 37.4 C and a pressure of 720 mmHg.
-
A sample of gas has a mass of 0.205 g. Its volume is 0.112 L at a temperature of 25 C and a pressure of 740 mmHg. Find its molar mass.
-
A 1.50-L mixture of helium, neon, and argon has a total pressure of 754 mmHg at 310 K. If the partial pressure of helium is 431 mmHg and partial pressure of neon is 211 mmHg, what mass of argon is...
-
business law A partner may actively compete with the partnership True False
-
A company provided the following data: Selling price per unit $80 Variable cost per unit $45 Total fixed costs $490,000 How many units must be sold to earn a profit of $122,500?
-
Suppose a 10-year, 10%, semiannual coupon bond with a par value of $1,000 is currently selling for $1,365.20, producing a nominal yield to maturity of 7.5%. However, it can be called after 4 years...

Study smarter with the SolutionInn App


