Use the standard enthalpies of formation from Appendix G to calculate the enthalpy change for each of
Question:
Use the standard enthalpies of formation from Appendix G to calculate the enthalpy change for each of the following reactions at 298.15 K and 1 atm. Label each as endothermic or exothermic.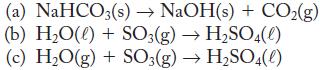
Transcribed Image Text:
(a) NaHCO3(s) → NaOH(s) + CO₂(g) (b) H₂O(l) + SO3(g) →H₂SO4(0) (c) H₂O(g) + SO3(g) → H₂SO4(l)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
a 13169 kJ en...View the full answer

Answered By

Robert Mwendwa Nzinga
I am a professional accountant with diverse skills in different fields. I am a great academic writer and article writer. I also possess skills in website development and app development. I have over the years amassed skills in project writing, business planning, human resource administration and tutoring in all business related courses.
4.90+
187+ Reviews
378+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Use the standard enthalpies of formation from Appendix G to calculate the enthalpy change for each of the following reactions at 298.15 K and 1 atm. Label each as endothermic or exothermic. (a) The...
-
The standard enthalpies of formation of ClO and ClO2 are 101 and 102 kJ/mol, respectively. Using these data and the thermodynamic data in Appendix C, calculate the overall enthalpy change for each...
-
The standard free energies of formation and the standard enthalpies of formation at 298 K for difluoroacetylene (C2F2) and hexafluorobenzene (C6F6) are For the following reaction: C6F6(g) 3C2F2(g) a....
-
Vito Co.'s next dividend is expected to be $4.50. Dividend growth is estimated at 20%, 15%, 8% for the following three years, and then stabilize to 2%. How much are you willing to pay to buy one...
-
5,000 lb/h of a saturated aqueous solution of (NH4)2SO4 at 80C is cooled to 30C. At equilibrium, what is the amount of crystals formed in lb/h. If during the cooling process, 50% of the water is...
-
The financial information below was taken from the annual financial statements of Crane Videos: \ table [ [ Current assets, 2 0 1 4 , 2 0 1 3 ] , [ Current liabilities,$ 9 7 , 4 0 0 , $ 9 4 , 3 0 0
-
What is the difference between a written option and a purchased option?
-
A General Power bond carries a coupon rate of 8%, has 9 years until maturity, and sells at a yield to maturity of 7%. (Assume annual interest payments.) a. What interest payments to bondholders...
-
Logan Manufacturing Co . warrants its products for one year. The estimated product warranty is 4 % of sales. Assume that sales were $ 7 0 0 , 0 0 0 for January. In February, a customer received...
-
Refer the following table. Focus Metals Inc. Comparative Balance Sheet Information: November 30 (millions of $) 2020 Cash 16 86 Accounts receivable (net). 386 232 60 53 Inventory Plant and equipment...
-
Use data in Appendix G to find the enthalpy of reaction for (a) CaCO3(s) CaO(s) + CO(g) (b) 2HI(g) + F2(g) 2HF(g) + I(s) (c) SF6(g) + 3HO() 6HF(g) + SO3(g)
-
Use standard enthalpies of formation to calculate the enthalpy change for each of the following reactions at 298.15 K and 1 atm. Label each as endothermic or exothermic. (a) The fermentation of...
-
The following information was taken from the annual report of ROM. The account balances are as of December 31, 2011. Cash.. $ 1,220 Accounts receivable.. 3,112 Merchandise inventory.. 966 Prepaid...
-
At March 31, account balances after adjustments for Vizzini Cinema are as follows: Account Balances Accounts Cash Supplies Equipment (After Adjustment) $11,000 4,000 50,000 Accumulated...
-
2. "A student holds a thin aluminum pie pan horizontally 2 m above the ground and releases it. Using a motion detector, she obtains the graph shown in Figure P3.12. Based on her measurements, (a)...
-
Mark has two sticks, 25 inches, and 20 inches. If he places them end-to-end perpendicularly, what two acute angles would be formed when he added the hypotenuse?
-
A wedding website states that the average cost of a wedding is $29,205. One concerned bride hopes that the average is less than reported. To see if her hope is correct, she surveys 36 recently...
-
2. (10 pts each) Use partial fractions decomposition and the tables to find the inverse z- transform of each of the following: a. X(z)= 6z-z z3-4z2-z+4 4z2 b. G(z)=- (z-1) (z-0.5) 3z +1 c. X(z) =...
-
Repeat Exercise 22.25, but use the multiversion timestamp ordering method.
-
D Which of the following is considered part of the Controlling activity of managerial accounting? O Choosing to purchase raw materials from one supplier versus another O Choosing the allocation base...
-
Describe the changes in a beaker containing water and butanol that you would observe along the path f j k in Figure 19.24b. How would you calculate the relative amounts of different phases present...
-
Describe the system at points a and c in Figure 19.25b. How would you calculate the relative amounts of different phases present at these points?
-
What information can be obtained from a tie line in a PZ phase diagram?
-
Green Lawn Company sells garden supplies. Management is planning its cash needs for the second quarter. The following information has been assembled to assist in preparing a cash budget for the...
-
eBook Question Content Area Comparison of Methods of Allocation Duweynie Pottery, Inc., is divided into two operating divisions: Pottery and Retail. The company allocates Power and General Factory...
-
TYBALT CONSTRUCTION Income Statement For Year Ended December 31 TYBALT CONSTRUCTION Income Statement For Year Ended December 31

Study smarter with the SolutionInn App


