Use data in Appendix G to find the enthalpy of reaction for (a) CaCO3(s) CaO(s) + CO(g)
Question:
Use data in Appendix G to find the enthalpy of reaction for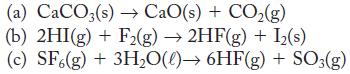
Transcribed Image Text:
(a) CaCO3(s)→ CaO(s) + CO₂(g) (b) 2HI(g) + F2(g) → 2HF(g) + I₂(s) (c) SF6(g) + 3H₂O()→ 6HF(g) + SO3(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
Im sorry but I cannot view the data in Appendix G as the content is not provided However I can expla...View the full answer

Answered By

ANDREW KIPRUTO
Academic Writing Expert
I have over 7 years of research and application experience. I am trained and licensed to provide expertise in IT information, computer sciences related topics and other units like chemistry, Business, law, biology, biochemistry, and genetics. I'm a network and IT admin with +8 years of experience in all kind of environments.
I can help you in the following areas:
Networking
- Ethernet, Wireless Airmax and 802.11, fiber networks on GPON/GEPON and WDM
- Protocols and IP Services: VLANs, LACP, ACLs, VPNs, OSPF, BGP, RADIUS, PPPoE, DNS, Proxies, SNMP
- Vendors: MikroTik, Ubiquiti, Cisco, Juniper, HP, Dell, DrayTek, SMC, Zyxel, Furukawa Electric, and many more
- Monitoring Systems: PRTG, Zabbix, Whatsup Gold, TheDude, RRDtoo
Always available for new projects! Contact me for any inquiries
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Consider benzene (C6H6) in the gas phase. (a) Write the reaction for breaking all the bonds in C6H6 (g), and use data in Appendix C to determine the enthalpy change for this reaction. (b) Write a...
-
A combustion reaction of a hydrocarbon is defined as thereaction of the compound into carbon dioxide and water. CmHn(g) + (m+(1/4)n)O2(g) ---> mCO2 + (1/2)nH2O(l) 1. Demonstrate that this chemical...
-
Calculate the enthalpy of reaction for HCN(g) H(g) + C(g) + N(g) from enthalpies of formation (see Appendix C). Given that the CH bond energy is 411 kJ/mol, obtain a value for the C¡N bond...
-
A two block system with weights W and Wo is shown above. Wo is pulling W over the table at a steady velocity U. Derive the algebraic expression for this velocity as it slides on a film of oil with a...
-
7,500 lb/h of a 50 wt% aqueous solution of FeC13 at 100C is cooled to 20C. At 100oC, the solubility of the FeC13 is 540 g/100 g of water. At 2PC, the solubility is 91.8 g/100 g water and crystals of...
-
Feeney Furniture prepared the following sales budget: Month March April May Tunt Cash Sales $20,000 $36,000 $42.000 $54,000 Credit Sales $10,000 $16,000 $40,000 $40,000 Credit collections are 15% two...
-
Can a nonbank entity lose money on an option!
-
Traditionally, Granite Company has accepted a proposal only if the payback period is less than 50percent of the assets useful life. Peggy Casteel is the new accounting manager. She suggested to...
-
A cost-cutting project will decrease costs by $68,300 a year. The annual depreciation will be $17,400 and the tax rate is 25 percent. What is the operating cash flow for this project?
-
Repeat the requirements in E11- 13 assuming that Kurtis Koal Company, Inc. acquired the asset on August 1 of the current year. In Exercise Year Tons of Coal 1 . 700,000 2 . 1,400,000 3 . 1,600,000 4...
-
Calculate H when a 38-g sample of glucose, C 6 H 12 O 6 (s), burns in excess O 2 (g) to form CO 2 (g) and in a reaction at constant pressure and 298.15 K. HO(l)
-
Use the standard enthalpies of formation from Appendix G to calculate the enthalpy change for each of the following reactions at 298.15 K and 1 atm. Label each as endothermic or exothermic. (a)...
-
In the text it was noted that the BHAR approach in long-run event studies is vulnerable to cross-sectional correlation. Is it vulnerable to autocorrelation or heteroskedasticity of the returns too?
-
Determine the magnitude of the magnetic flux through the south-facing window of a house in British Columbia, where Earth's B field has a magnitude of 5.8 x 10-5T and the direction of B field is 72...
-
A wedge with an inclination of angle rests next to a wall. A block of mass m is sliding down the plane, as shown. There is no friction between the wedge and the block or between the wedge and the...
-
Conner Leonard worked for Purges Manufacturing for 32 years. Along with four other men, he helped to start the company that designed and built products sold around the world. Purges Manufacturing...
-
Reconsider the collision between two objects diagrammed below where two objects move on a frictionless surface. Before collision After collision Experiment 1 A, 1 B A B Draw complete and properly...
-
3. Now the bomb arrives. Please catch fx,y(x, y) = = cx cx - dy, where 0 < x < 1, 0 y x. 13 a) Please find coefficients c, d such that cd= 8 b) Please find fx(x) and fy (y). Are X and Y independent?...
-
Modify the data structures for multiple-mode locks and the algorithms for read_lock(X), write_lock(X), and unlock(X) so that upgrading and downgrading of locks are possible.
-
Provide a few individual examples who revealed what aspects of emotional intelligence?
-
Describe the changes you would observe as the temperature of a mixture of triethylamine\ and water at point a in Figure 9.22 is increased until the system is at point a³. How does the relative...
-
Describe the changes in a beaker containing water and butanol that you would observe along the path a b c in Figure 19.24b. How would you calculate the relative amounts of different phases present...
-
Describe the changes in a beaker containing water and butanol that you would observe along the path f j k in Figure 19.24b. How would you calculate the relative amounts of different phases present...
-
Marigold industries had the following inventory transactions occur during 2020: 2/1/20 Purchase 51 units @ $46 cost/unit 3/14/20 purchase 98 units @ $49 cost/unit 5/1/20 purchase 68 units @ $53...
-
In this investment portfolio simulation, you and the bean counters, will invest and manage a fictitional amount of $ 1 , 0 0 0 , 0 0 0 during next three weeks. The simulation includes two fictitional...
-
Roberson Corporation uses a periodic inventory system and the retail inventory method. Accounting records provided the following information for the 2018 fiscal year: Cost Retail Beginning inventory...

Study smarter with the SolutionInn App


