Describe the changes you would observe as the temperature of a mixture of triethylamine and water at
Question:
Figure 9.22
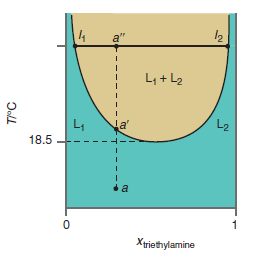
Transcribed Image Text:
a" L4 + L2 L2 a' 18.5 da Xtriethylamine T/°C
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 58% (12 reviews)
At point a the two components are completely miscible and phase separation does not oc...View the full answer

Answered By

Balaji Adireddi
I completed my bachelor in normal state College. Because of my hardwork i git addmission in NIT ROURKELA for masters and keep up the same spirit now I'm doing doctorate in IIT HYDERABAD
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A red ball is placed at point A in FIGURE P34.44. a. How many images are seen by an observer at point O?b. What are the (x, y) coordinates of each image? 3.0 m 2.0 m 3.0 m 1.0 m FIGURE P34.44
-
What would be the final temperature of a mixture of 50 g of 20C water and 50 g of 40C water?
-
(a) Find the electric potential at point P in FIGURE 20-33. (b) Suppose the three charges shown in Figure 20-33 are held in place. A fourth charge, with a charge of +4.82 C and a mass of 2.33 g, is...
-
Cost information for Lake County Library is as follows. In addition to directly traceable costs, the library incurred $24,000 for a building lease. REQUIRED A. Allocate to departments any costs that...
-
Is it better to minimize the cost of the inventory with a method like EOQ or to order only to meet demand?
-
As far as alcoholic beverages go, what does the phrase "value for money" mean to you ?
-
Why might an organization enter into a know-how agreement rather than another form of entry strategy? LO.1
-
The R&D division of Mozy Corp. has just developed a chemical for sterilizing the vicious Brazilian killer bees which are invading Mexico and the southern United States. The president of Mozy is...
-
When preparing consolidated financial statements, which of the following is a subtraction in the calculation of cash flows from operating activities under the indirect method? Select one: a....
-
Open orbits an open orbit in monovalent tetragonal metal connects opposite faces of the boundary of a Brillouin zone. The faces are separated by G = 2 x 10 8 cm 1 . A magnetic field B = 10 3 gauss =...
-
Describe the changes you would observe as the temperature of a mixture of phenol and water at point a in Figure 9.21 is increased until the system is at point a². How does the relative amount of...
-
Describe the changes in a beaker containing water and butanol that you would observe along the path a b c in Figure 19.24b. How would you calculate the relative amounts of different phases present...
-
Suppose a firm had the following assets at the end of a year: And suppose the firm had sales of $100,000. Using the percentage of sales methods and using this year as the base year, what are the...
-
Skinovations needs to put together a Production schedule for next week and has asked its marketing team to give its forecasts for next week's sales. The team has used two different forecasting...
-
If a potential leader viewed her least preferred co-worker in favorable terms, how would Fiedler's Model describes this leader?
-
You have just been hired as a financial analyst for Lydex Company, a manufacturer of safety helmets. Your boss has asked you to perform a comprehensive analysis of the company s financial statements,...
-
For our first discussion you should locate a research article in which a quantitative study is reported. This article should not be a theoretical article or a methods article, but should describe...
-
A box is separated by a partition which divides its volume in the ration of 3:1. the larger portion of the box contains 1000 molecules of Ne gas; the smalled portion contains 100 molecules of He gas....
-
Find any horizontal or vertical asymptotes. f(x) = 4x 2x6
-
Which of the companies has the lowest accounts receivable turnover in the year 20X2? a. Company A. b. Company B. c. Company C. d. CompanyD. 20X1 20X2 Credit Sales Average Receivables Balance $1.0...
-
Suggest the pressure and temperature at which 1.0 mol of (a) NH 3 , (b) Xe, (c) He will be in states that correspond to 1.0 mol H 2 at 1.0 atm and 25C.
-
The equation of state of a certain gas is given by p = RT/V m + (a + bT)/V 2 m , where a and b are constants. Find (V/T) p .
-
Use the van der Waals parameters for chlorine to calculate approximate values of (a) The Boyle temperature of chlorine and (b) The radius of a Cl 2 molecule regarded as a sphere.
-
Chapter o Homew ebook 50,000-unit production quantity: $ 227,049 7 70,000-unit production quantity: $ 66,751 d. In addition to mean profit, what other factors should FTC consider in determining a...
-
Diamond makes downhill ski equipment. Assume that comic has offered to produce ski poles for Diamond for $20 per pair Diamond needs 200,000 pairs of poles per period Diamond can only avoid 5150,000...
-
17? Which of the following statement is true Select one: a. All evidence must have the same level of reliability b. All evidence must have the same level of persuasiveness C. All are false d....

Study smarter with the SolutionInn App


