Use the VSEPR model to predict the bond angles around each central atom in the following Lewis
Question:
Use the VSEPR model to predict the bond angles around each central atom in the following Lewis structures (left). Note that the drawings do not necessarily depict the bond angles correctly.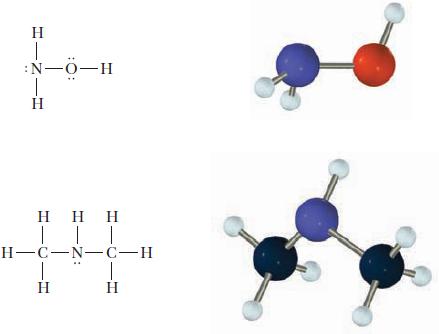
Transcribed Image Text:
H :N-0-H I | | | H-C-N-C-H T T
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
The VSEPR Valence Shell Electron Pair Repulsion model is used to predict the geometry of a molecule ...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Use the VSEPR model to predict the bond angles around each central atom in the following Lewis structures (benzene rings are frequently pictured as hexagons, without the letter for the carbon atom at...
-
Use the VSEPR model to predict the bond angles around each central atom in the following Lewis structures. Note that the drawings do not necessarily depict the bond angles correctly. (a) H :0: H | ||...
-
For each of the following molecules, complete the Lewis structure and use the VSEPR model to determine the bond angles around each central atom. Note that the drawings are only skeleton structures...
-
The cable of the 1800 kg elevator cab in Figure snaps when the cab is at rest at the first floor, where the cab bottom is a distance d = 3.7 m above a spring of spring constant k = 0.15 MN/m. A...
-
Applying Percentage of Sales the balance sheet for the Heir Jordan Corporation follows. Based on this information and the income statement in the previous problem, supply the missing information...
-
At the end of the current year, the accounts receivable account of Glenns Nursery Supplies has a debit balance of $390,000. Credit sales are $2,800,000. Record the end-of-period adjusting entry on...
-
Think of where you work, or where you have worked, and identify three activities where global thinking influences local action. What are the local HR implications of this?? LO1
-
Bob Night opened "The General's Favorite Fishing Hole." The fishing camp is opened from April through September and attracts many famous college basketball coaches during the off-season. Guests...
-
A manufacturer of ovens sells them for $1,290.00 each. The variable costs are $860.00 per unit. The manufacturer's factory has annual fixed costs of $1,815,000.00. Given the expected sales volume of...
-
Write a Lewis structure for each of the following species. Indicate all of the bond angles as predicted by the VSEPR model. Deduce the skeleton structure from the way each formula is written. (a) SO...
-
For each of the following molecules, complete the Lewis structure and use the VSEPR model to determine the bond angles around each central atom. Note that the drawings are only skeleton structures...
-
Repeat Problem 8 for a disk of uniform surface charge density s.
-
Prove (11.32) . E (Yi,k | Zi = 0, = e) = E (Yi,k | i = 1, = e) = E (Yi,k | Ti = e), k = 1,2. (11.32)
-
University Medical Center needs to move from its existing facility to a new and larger facility five miles away from its current location. Due to construction delays, however, much of the new...
-
Calculate the base value or lump sum for each of the single and married filing jointly 2016 brackets given in Table 6.4. Table 6.4 ITABLE 6.4 Corporate Income Brackets and Tax Rates, 2015 Taxable...
-
Show that staged column diameter is proportional to (feed rate) \({ }^{1 / 2}\) and to \((1+\mathrm{L} / \mathrm{D})^{1 / 2}\).
-
An atmospheric column with 25 real stages is operating with a pressure drop of 0.6 in. of water per stage. Assume pressure drop in the condenser and the reboiler is \(1.2 \mathrm{in}\). of water...
-
Cocaine metabolism in rats can be studied by injecting the drug and periodically withdrawing blood to measure levels of metabolites by HPLC-mass spectrometry. For quantitative analysis, isotopically...
-
Anne is employed by Bradley Contracting Company. Bradley has a $1.3 million contract to build a small group of outbuildings in a national park. Anne alleges that Bradley Contracting has discriminated...
-
The shaft of radius c is subjected to a distributed torque t, measured as torque/length of shaft. Determine the angle of twist at end A. The shear modulus is G. t= to(1 + ()) to 2to
-
The A-36 steel bolt is tightened within a hole so that the reactive torque on the shank AB can be expressed by the equation t = (kx 2 ) N m/m, where x is in meters. If a torque of T = 50 N m is...
-
The A-36 steel bolt is tightened within a hole so that the reactive torque on the shank AB can be expressed by the equation t = (kx 2 /3) N m/m, where x is in meters. If a torque of T = 50 N m is...
-
QUESTION 3 A business owns seven flats rented out to staff at R500 per month. All flats were tenanted Ist january 21 months rent was in arrears and as at 31st December 14 months' rent wa Identify the...
-
1. 2. 3. Select the Tables sheet, select cells A6:B10, and create range names using the Create from Selection button [Formulas tab, Defined Names group]. Select cells B1:F2 and click the Name box....
-
Tropical Rainwear issues 3,000 shares of its $18 par value preferred stock for cash at $20 per share. Record the issuance of the preferred shares. (If no entry is required for a particular...

Study smarter with the SolutionInn App


