Use the VSEPR model to predict the bond angles around each central atom in the following Lewis
Question:
Use the VSEPR model to predict the bond angles around each central atom in the following Lewis structures. Note that the drawings do not necessarily depict the bond angles correctly.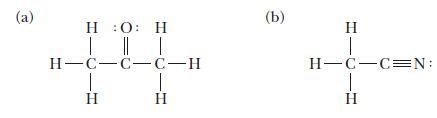
Transcribed Image Text:
(a) H :0: H | || | H-C-C-C-H H T H (b) H H-C-C=N: H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
a 1095 degrees around e...View the full answer

Answered By

AJIN kuriakose
I have completed B.Tech in Electrical Engineering & Masters in Power & Control From one of the best universities in India. I got the 99.05 percentile in the Gate Electrical Engineering Exam. I can Help students solving assignments in Electrical subjects like Power Electronics, Control system, Analog, Network Theory & Engineering Mathematics. Clear your fundamentals and develop problem-solving skills and analytical skills to crack the exam.
Get guidance and the opportunity to learn from experienced...
I can provide tuition for Electrical engineering subjects (Power Electronics, Digital electronics, Network Theory, Control System & Engineering Mathematics). The toughest subject of Electrical engineering can be made simple in online classes...
I can also solve it.
1 .I can help you with your assignments or exams or quiz or tutoring.
2. Very strict to the deadlines.
Message me for any help in assignments, live sessions. I am here to help students for all assignments, tests and exams and I will make sure you always get _95% In your subject.
Contact me in solution inn for any help in your semester, projects and for many more things . Also feel free to contact me through solution inn and for any advise related to tutoring and how it works here.thank you.
5.00+
5+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Use the VSEPR model to predict the bond angles around each central atom in the following Lewis structures (benzene rings are frequently pictured as hexagons, without the letter for the carbon atom at...
-
Use the VSEPR model to predict the bond angles around each central atom in the following Lewis structures (left). Note that the drawings do not necessarily depict the bond angles correctly. H :N-0-H...
-
For each of the following molecules, complete the Lewis structure and use the VSEPR model to determine the bond angles around each central atom. Note that the drawings are only skeleton structures...
-
The controller of Northwest Hardware has just received two forecasts for sales in the Montana District for the coming year. Based on an econometric analysis of consumer spending and economic trends,...
-
The switch in the circuit in figure has been closed for a long time and is opened at t = 0. Find i(t) for t > 0, using Laplacetransforms. 4 0 16 340 i(t) 6 v(+ 24 V
-
What is the qualitative characteristic of comparability? Why is it important in preparing financial statements?
-
Operation Crossroads was a 1946 military exercise in which atomic bombs were detonated over empty target ships in the Pacific 0cean.The Navy assigned sailors to wash down the test ships immediately...
-
XS Supply Company is developing its annual financial statements at December 31, 2014. The statements are complete except for the statement of cash flows the complete comparative balance sheets and...
-
The balance sheet of the Captain Fishing Inc. is attached. During 2017, the following events occurred. 1. On January 10, sold merchandise on account to Rayms $8800 and Fischer $7500. Terms 2/10,...
-
Use the VSEPR model to predict the shape of the following species. (a) BeF 2 (b) SF 6 (c) SiH 4 (d) FCN (e) BeF 3
-
Assuming that the molecular orbital diagram shown in Figure 10.40 is correct for heteronuclear diatomic molecules containing elements that are close to each other in the periodic table, write a...
-
Demonstrate that the two elliptic curves of Figure 10.4 each satisfy the conditions for a group over the real numbers. 4 2 0 -21 -4 -2 P -1 1 (a) y = x3 . 2 - (P + Q) (P + Q) 3 4 5
-
Perpetual Inventory Control Record Description: M & B Supreme Date Purchase Received Issued Sales Units Unit Cost June 1 Balance forward 3 $10.00 4 2 6 8 9 $10.50 9 12 32 3 6 2 4 15 6 10 $11.00 18 20...
-
A rectangular footing of size 4m by 5m is founded at 2m below ground level in a uniform deposit of saturated clay. The footing is designed to support a total vertical load of 8000 kN inclusive of the...
-
P6.2 At the start of Tom Stoppard's "Rosencrantz and Guildenstern are dead" 1, Rosencrantz finds a coin. Guildenstern watches as Rosencrantz repeatedly tosses the coin and every time it comes down...
-
For the data: 9 5 10 7 9 10 11 8 12 769 a) Compute the z-score for the raw score of 10 b) Find the raw score that corresponds to z=+1.22
-
(11%) Problem 7: After a bad thunderstorm, a loose power line comes to rest on a parked van. The van is insulated from the ground by its tires, and accumulates an electric charge of Q = 0.0012...
-
Write a line notation and two reduction half-reactions for each cell pictured above. Fe Ag Pb Pb KOH(aq) K2SO4 (aq) H,S04(aq) FeO(s) Ag 0(s) PbSO4(s) PbSO,(s) PbO2(s) Galvanic cells for Problem 13.8
-
Ex. (17): the vector field F = x i-zj + yz k is defined over the volume of the cuboid given by 0x a,0 y b, 0zc, enclosing the surface S. Evaluate the surface integral ff, F. ds?
-
The aluminum strut is 10 mm thick and has the cross section shown. If it is subjected to a shear of V = 150 N, determine the shear flow at points A and B. 10 mm 40 mm 10 mm t+-40 mm- 30 mm 30 mm 10...
-
The aluminum strut is 10 mm thick and has the cross section shown. If it is subjected to a shear of V = 150 N, determine the maximum shear flow in the strut. 10 mm 40 mm 10 mm tr-40 mm- 30 mm 30 mm...
-
The beam is subjected to a shear force of V = 50 kip. Determine the shear flow at points A and B. 1 in. 5 in. 1 in. 5 in. 9 in. 1 in. B 12 in.
-
An 8%, 30-year semi-annual corporate bond was recently being priced to yield 10%. The Macaulay duration for this bond is 10.2 years. What is the bonds modified duration? How much will the price of...
-
Question 7 of 7 0/14 W PIERDERY Current Attempt in Progress Your answer is incorrect Buffalo Corporation adopted the dollar value LIFO retail inventory method on January 1, 2019. At that time the...
-
Cost of debt with fees . Kenny Enterprises will issue a bond with a par value of $1,000, a maturity of twenty years, and a coupon rate of 9.9% with semiannual payments, and will use an investment...

Study smarter with the SolutionInn App


