Using Figure 8.18, suggest compounds from Group 1A to put in fireworks that would burn (a) Red.
Question:
Using Figure 8.18, suggest compounds from Group 1A to put in fireworks that would burn
(a) Red.
(b) Yellow.
Figure 8.18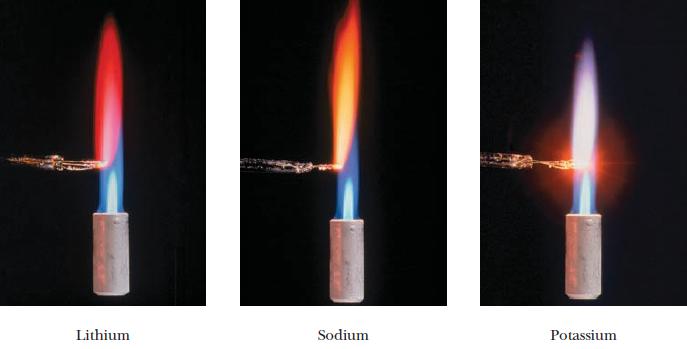
Transcribed Image Text:
Lithium Sodium Potassium
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)
The image youve provided labeled as Figure 818 shows three separate flames resulting from the burnin...View the full answer

Answered By

S Mwaura
A quality-driven writer with special technical skills and vast experience in various disciplines. A plagiarism-free paper and impeccable quality content are what I deliver. Timely delivery and originality are guaranteed. Kindly allow me to do any work for you and I guarantee you an A-worthy paper.
4.80+
27+ Reviews
73+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
For the following two graphics, provide the specified information below for each. Inverse Demand: P= 43.75 - .00625 Q; MR = 43.75 - 0.0125 Q 25 20 15 $ per unit 10 10 5 0 MC 500 1000 1500 ATC 2000 -...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-6. On December 12, Irene purchased the building where her store is located. She paid...
-
Carol Harris, Ph.D, CPA, is a single taxpayer and she lives at 674 Yankee Street, Durham, NC 27409. Her Social Security number is 793-52-4335. Carol is an Associate Professor of Accounting at a local...
-
PacTec Luggage Shop is a small retail establishment located in a large shopping mall. This shop has implemented the following procedures regarding inventory items: a. Since the display area of the...
-
An object is projected vertically from the surface of the earth. Show that the maximum height reached by the object is H = REH / (RE H), where H is the height that it would reach if the...
-
As a soap bubble evaporates, it appears black just before it breaks. Explain this phenomenon in terms of the phase changes that occur on reflection from the two surfaces of the soap film.
-
how to interpret logistic regression results
-
A temperature control system for a distillation column is shown in figure. The temperature T of a tray near the top of the column is controlled by adjusting the reflux flow rate R. Draw a block...
-
Smithen Company, a wholesale distributor, has been operating for only a few months. The company sells three products- sinks, mirrors, and vanities. Budgeted sales by product and in total for the...
-
Reba Dixon is a fifth-grade school teacher who earned a salary of $38,000 in 2020. She is 45 years old and has been divorced for four years. She receives $1,200 of alimony payments each month from...
-
State the reactivity trend down the group for the elements in the following groups. (a) 1A (b) 2A (c) 7A
-
Write the electron configurations of the following elements after finding their locations in the periodic table. (a) P (b) Sr (c) Sm (d) Ra
-
You are from Corporate Headquarters. As a member of the HR department, you are responsible for managing international assignments. You are to present a 10-minute summary of the key aspects of...
-
1. Why do companies that choose to open subsidiaries in other countries have different HR responsibilities? 2. How has globalization allowed companies to become "global companies" more easily? 3....
-
Is Kroger's innovation Product-related or process-related? Do the innovations tend to be incremental or radical? https://www.thekrogerco.com/about-kroger/our-business/ Kroger Co. opens new spoke in...
-
Define what is Process Mapping/Value Stream Mapping How do you apply process mapping methodology? What are the advantages of leaders using process mapping Identify a real world business...
-
What role do formalized processes and protocols play in highly structured organizations, and how can organizations balance the need for structure with the imperative for flexibility and innovation ?
-
In what ways do decision-makers balance quantitative data with qualitative insights to optimize complex strategic choices, especially in high-stakes business environments where traditional metrics...
-
Using the values of the local Nusselt number given in Figure 6-11, obtain values for the average Nusselt number as a function of the Reynolds number. Plot the results as log Nu versus log Re, and...
-
Give the products of the following reaction, where T is tritium: dioldehydrase Ad- CH CH3C-COH CoIII) coenzyme B12
-
a. Determine the total collisional frequency for CO 2 at 1 atm and 298 K. b. At what temperature would the collisional frequency be 10% of the value determined in part (a)? For CO 2 , = 5.2 10 19 m...
-
a. A standard rotary pump is capable of producing a vacuum on the order of 10 3 Torr. What is the single-particle collisional frequency and mean free path for N 2 at this pressure and 298 K? b. A...
-
Determine the mean free path for Ar at 298 K at the following pressures: a. 0.500 atm b. 0.00500 atm c. 5.00 10 6 atm For Ar, = 3.6 10 19 m 2 (see Table 33.1) and M = 0.040 kg mol 1 .
-
Al preparar el estado de resultados pro forma, cules de las siguientes partidas se deducen de las utilidades brutas para llegar a las ganancias despus de impuestos? Pregunta de seleccin mltiple....
-
Lawson Inc. is expanding its manufacturing plant, which requires an investment of $4 million in new equipment and plant modifications. Lawson's sales are expected to increase by $3 million per year...
-
20 On January 1, Year 1, X Company purchased equipment for $80,000. The company estimates that the equipment will have a useful life of 10 years and a residual value of $5,000. X Company depreciates...

Study smarter with the SolutionInn App


