Using Table 9.4, calculate the energy required to break all of the bonds in one mole of
Question:
Using Table 9.4, calculate the energy required to break all of the bonds in one mole of the following compounds.
(a) CH2CF2
(b) N2H4
Table 9.4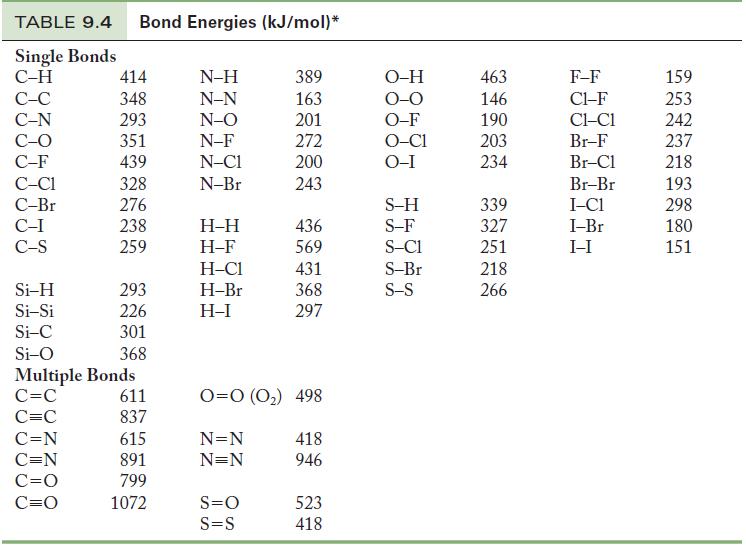
Transcribed Image Text:
TABLE 9.4 Bond Energies (kJ/mol)* Single Bonds C-H C-C C-N C-O C-F C-C1 C-Br C-I C-S Si-H Si-Si Si-C Si-O 414 348 293 351 439 328 276 238 259 C=N C=N C=O C=O 293 226 301 368 Multiple Bonds C=C C=C 611 837 615 891 799 1072 N-H N-N N-O N-F N-C1 N-Br H-H H-F H-C1 H-Br H-I 389 163 201 272 200 243 S=O S=S 436 569 431 368 297 0=0 (0₂) 498 N=N N=N 418 946 523 418 O-H O-O O-F O-C1 O-I S-H S-F S-C1 S-Br S-S 463 146 190 203 234 339 327 251 218 266 F-F C1-F CLC1 Br-F Br-Cl Br-Br I-C1 I-Br I-I 159 253 242 237 218 193 298 180 151
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
a dissociation bonds broken EAH bonds formed diss...View the full answer

Answered By

Shebla K
I am an MBA graduate having experience as an Assistant Professor at University level for two years. I always prepare well for a class as I believe that only if you become an ocean you can give a bucket of water. Being a teacher was not only my profession but also my passion.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Using Table 9.4, calculate the energy required to break all of the bonds in one mole of the following compounds. (a) NH 3 (b) CH 3 OH Table 9.4 TABLE 9.4 Bond Energies (kJ/mol)* Single Bonds C-H...
-
The specific heat capacity of silver is 0.24 JoC-1g-1. a. Calculate the energy required to raise the temperature of 150.0 g Ag from 273 K to 298 K. b. Calculate the energy required to raise the...
-
Calculate the energy required to assemble the array of charges shown in Figure P25.34, where a = 0.200 m, b = 0.400 m, and q = 6.00 +C.
-
Describe An Analysis of the relationship between Employee Engagement, Satisfaction, and Organizational Performance at Enterprise Mobility
-
Using atomic weight, crystal structure, and atomic radius data tabulated inside the front cover, compute the theoretical densities of lead, chromium, copper, and cobalt, and then compare these values...
-
A spherical capacitor has inner radius a and outer radius d. Concentric with the spherical conductors and lying between them is a spherical shell of outer radius c and inner radius b. If the regions...
-
What do we mean by work motivation, and how does it relate to individual performance in the workplace? Why is individual work motivation important to organizational success? (L01)
-
James Albemarle created a trust fund at the beginning of 2016. The income from this fund will go to his son Edward. When Edward reaches the age of 25, the principal of the fund will be conveyed to...
-
Exercise 11-8A Effect of issuing common stock on the balance sheet LO 11-4 Newly formed S&J Iron Corporation has 145,000 shares of $7 par common stock authorized. On March 1, Year 1, S&J Iron issued...
-
Which molecule has the most polar bond: N 2 , BrF, or ClF? Use an arrow to show the direction of polarity in each bond.
-
Using Table 9.4, calculate an approximate enthalpy change for (a) The reaction of molecular hydrogen (H 2 ) and molecular oxygen (O 2 ) in the gas phase to produce 2 mol water vapor. (b) The reaction...
-
A bubble-point liquid feed is to be distilled as shown in Figure. Use the Edmister group method to estimate the mole-fraction compositions of the distillate and bottoms. Assume initial overhead and...
-
A release has been planned with 5 sprints. The team, for the sake of convenience, has decided to keep the sprint duration open. Depending on how much they commit and achieve, they decide to wrap up...
-
Task 3: Reach-truck management 3 Explain why battery-powered reach truck activities at PAPFS are unsatisfactory. Note: You should support your answer, where applicable, using relevant information...
-
Exercise 6: Black Pearl, Inc., sells a single product. The company's most recent income statement is given below. Sales $50,000 Less variable expenses Contribution margin Less fixed expenses Net...
-
Your maths problem x+3x-3
-
Spencer is a 10-year-old boy who has been living in a family-style therapeutic group home for one year. He was removed from his mother's care due to neglect from her drug use and the resulting legal...
-
Intramitochondrial ATP concentrations are about 5 mM, and phosphate concentration is about 10 mM. If ADP is five times more abundant than AMP, calculate the molar concentrations of ADP and AMP at an...
-
Define cultural intelligence. Cite the books or journal articles you found in Capella's library. Explain why cultural intelligence is important for HR practitioners and other organizational managers.
-
A shaft is made of an aluminum alloy having an allowable shear stress of Ï allow = 100 MPa. If the diameter of the shaft is 100 mm, determine the maximum torque T that can be transmitted. What...
-
The solid shaft of radius r is subjected to a torque T. Determine the radius r' of the inner core of the shaft that resists one-quarter of the applied torque (T/4). Solve the problem two ways:...
-
The solid shaft of radius r is subjected to a torque T. Determine the radius r of the inner core of the shaft that resists one-half of the applied torque (T>2). Solve the problem two ways: (a)...
-
The Work in Process inventory account of a manufacturing company shows a balance of $2,400 at the end of an accounting period. The job cost sheets of the two uncompleted jobs show charges of $400 and...
-
1. An investor buys a three-year bond with a 5% coupon rate paid annually. The bond, with a yield-tomaturity of 3%, is purchased at a price of 105.657223 per 100 of par value. Assuming a 5bp change...
-
Describe how the following affect the valuation of PPE. a) Cash Discounts b) Deferred Payment Contracts

Study smarter with the SolutionInn App


