Using Table 9.4, calculate an approximate enthalpy change for (a) The reaction of molecular hydrogen (H 2
Question:
Using Table 9.4, calculate an approximate enthalpy change for
(a) The reaction of molecular hydrogen (H2) and molecular oxygen (O2) in the gas phase to produce 2 mol water vapor.
(b) The reaction of carbon monoxide and molecular oxygen to form 2 mol carbon dioxide.
Table 9.4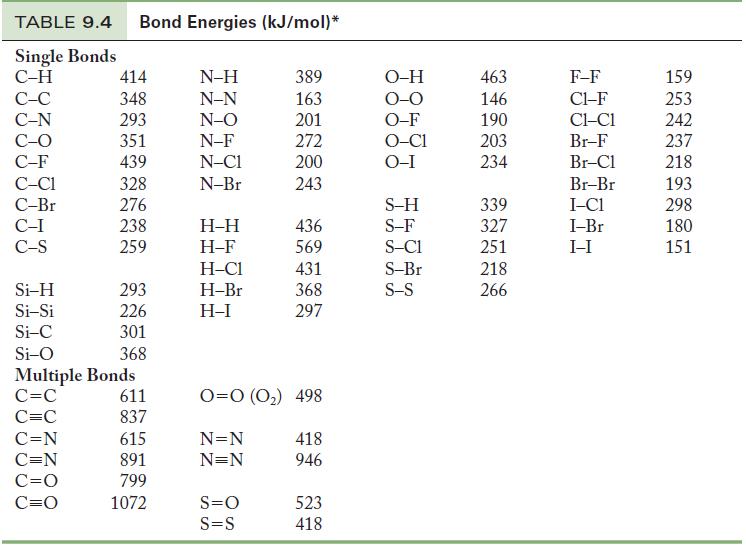
Transcribed Image Text:
TABLE 9.4 Bond Energies (kJ/mol)* Single Bonds C-H C-C C-N C-O C-F C-C1 C-Br C-I C-S Si-H Si-Si Si-C Si-O 414 348 293 351 439 328 276 238 259 C=N C=N C=O C=O 293 226 301 368 Multiple Bonds C=C C=C 611 837 615 891 799 1072 N-H N-N N-O N-F N-C1 N-Br H-H H-F H-C1 H-Br H-I 389 163 201 272 200 243 S=O S=S 436 569 431 368 297 0=0 (0₂) 498 N=N N=N 418 946 523 418 O-H O-O O-F O-C1 O-I S-H S-F S-C1 S-Br S-S 463 146 190 203 234 339 327 251 218 266 F-F C1-F CLC1 Br-F Br-Cl Br-Br I-C1 I-Br I-I 159 253 242 237 218 193 298 180 151
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
a 4...View the full answer

Answered By

Amit Choudhary
I'm new in this profession regarding online teaching but previously i used to teach students near my college. I am teaching on online platform since last year and got good support from the students. I'm teaching on platforms like chegg and vedantu and also at my home in free time.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
List major external and internal customer groups of apple Explain the value exchange for each customer group listed of apple
-
Use Table 9.4 to calculate an approximate enthalpy change for (a) The combustion of 1 mol C 2 H 4 in excess molecular oxygen to form gaseous water and CO 2 . (b) The reaction of 1 mol formaldehyde, H...
-
Solid oxide fuel cells (SOFC) have been proposed as an alternative energy technology for use in large stationary power applications (1 to 10MWof electrical power). These devices have an ion...
-
If Converse introduced a new SMART WATCH for Men and Women, should they launch the product using Market Skimming or Marketing Penetration? Explain answer
-
Zirconium has an HCP crystal structure and a density of 6.51 g/cm 3 . (a) What is the volume of its unit cell in cubic meters? (b) If the c/a ratio is 1.593, compute the values of c and a.
-
A spherical capacitor has inner radius a?and outer radius b?and filled with an inhomogeneous dielectric with ? = ? o k/r 2 . Show that the capacitance of the capacitor is 4xE,k
-
What assumptions does Maslows Hierarchy of Needs make about human motivation? How can managers use this theory to motivate associates? (L01)
-
In 2011, home prices and mortgage rates dropped so low that in a number of cities the monthly cost of owning a home was less than renting. The following data show the average asking rent for 10...
-
Jasper Company has 55% of its sales on credit and 45% for cash. All credit sales are collected in full in the first month following the sale. The company budgets sales of $522,000 for April, $532,000...
-
Using Table 9.4, calculate the energy required to break all of the bonds in one mole of the following compounds. (a) CH 2 CF 2 (b) N 2 H 4 Table 9.4 TABLE 9.4 Bond Energies (kJ/mol)* Single Bonds...
-
The equation for the combustion of gaseous methanol is Using the bond dissociation enthalpies, estimate the enthalpy change for this reaction. 2CH3OH(g) + 302(g) 2CO(g) + 4HO(g)
-
How do rooms demand forecasts affect the selling prices of a lodging operation's guest rooms?
-
In a fixed time and budget project, the customer wants the development of a core component to be based on agile practices, as the final scope of the requirement has not yet been fully developed. The...
-
1. Ace Pizzeria, a manufacturer of frozen pizzas, computes its predetermined overhead rate annually based on machine hours. At the beginning of the year, the company estimated that 165,000 machine...
-
From a group of 13 boys and 9 girls, a committee of 5 students is chosen at random. a. The probability that all 5 members on the committee will be girls is (Type an integer or a simplified fraction.)...
-
On December 1, 2020, Cream Ale Ltd. receives $1,800 in advance for an agreement to brew beer during the months of December, January, and February. What is the revenue recognized under accrual...
-
A survey shows that of 100 nurses, 75 play at least soccer, 95 play at least softball, and 50 play both soccer and softball. Is this possible? Step one: Understand the problem and organize the given...
-
Of the various oxidation reactions in glycolysis and the citric acid cycle, the only one that does not involve NAD+ is the succinate dehydrogenase reaction. What would G be for an enzyme that...
-
During 2012, Cheng Book Store paid $483,000 for land and built a store in Georgetown. Prior to construction, the city of Georgetown charged Cheng $1,300 for a building permit, which Cheng paid. Cheng...
-
The joint is made from three A992 steel plates that are bonded together at their seams. Determine the displacement of end A with respect to end B when the joint is subjected to the axial loads. Each...
-
The rigid link is supported by a pin at A and two A-36 steel wires, each having an unstretched length of 12 in. and cross-sectional area of 0.0125 in 2 . Determine the force developed in the wires...
-
The 2014-T6 aluminum rod has a diameter of 0.5 in. and is lightly attached to the rigid supports at A and B when T 1 = 70°F. Determine the force P that must be applied to the collar so that, when...
-
A project will generate annual cash flows of $237,600 for each of the next three years, and a cash flow of $274,800 during the fourth year. The initial cost of the project is $749,600. What is the...
-
You want to invest annual amounts over the next 15 years. If your goal is to have $15,000 at the end of that time and if you can earn 8 percent on your invested funds, how much do you need to invest...
-
please explain thoroughly how to do in excel 1. Find the number of units to ship from each factory to each customer that minimizes total cost

Study smarter with the SolutionInn App


