Use Table 9.4 to calculate an approximate enthalpy change for (a) The combustion of 1 mol C
Question:
Use Table 9.4 to calculate an approximate enthalpy change for
(a) The combustion of 1 mol C2H4 in excess molecular oxygen to form gaseous water and CO2.
(b) The reaction of 1 mol formaldehyde, H2CO, with molecular hydrogen to form gaseous methanol (CH3OH).
Table 9.4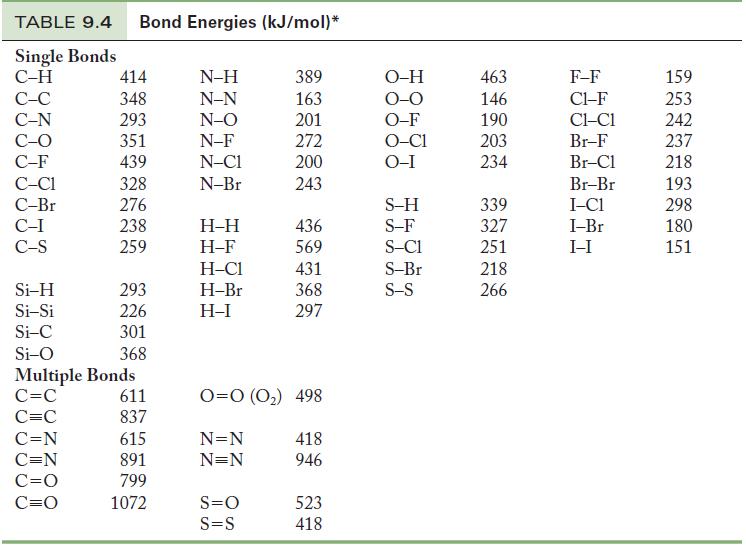
Transcribed Image Text:
TABLE 9.4 Bond Energies (kJ/mol)* Single Bonds C-H C-C C-N C-O C-F C-C1 C-Br C-I C-S Si-H Si-Si Si-C Si-O 414 348 293 351 439 328 276 238 259 C=N C=N C=O C=O 293 226 301 368 Multiple Bonds C=C C=C 611 837 615 891 799 1072 N-H N-N N-O N-F N-C1 N-Br H-H H-F H-C1 H-Br H-I 389 163 201 272 200 243 S=O S=S 436 569 431 368 297 0=0 (0₂) 498 N=N N=N 418 946 523 418 O-H O-O O-F O-C1 O-I S-H S-F S-C1 S-Br S-S 463 146 190 203 234 339 327 251 218 266 F-F C1-F CLC1 Br-F Br-Cl Br-Br I-C1 I-Br I-I 159 253 242 237 218 193 298 180 151
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a 1...View the full answer

Answered By

Sachin Singh
Dear Students,
I am Sachin, an M.Tech from IIT Delhi with several years of industrial experience in the software field. I am here to solve your problems, clear your doubts on that topic you find laborious, in the simplest of ways possible. I will make you understand the toughest of concepts with the easiest of approaches. You are going to find them very simple once we learn together.
I have spent years, have put in hundreds of hours in mastering my subjects. So, let me save you a lot of time understanding these subjects and solving the problems in an effortless fashion.
I hold a deep understanding of programming concepts, data structures, algorithms, digital electronics, discrete mathematics, etc.
I have varied experience in education and tutoring right from my schooling days. From the very start, I have been involved in teaching my fellow batch mates, juniors and kids around my neighborhood to help them with their assignments, complex problems and understanding any topic. People find my ways fun, engaging and interesting.
Teaching Style I follow :
Strong focus on the "why" in addition to the "what" while solving a problem or explaining a topic.
Root cause analysis for any problem/topic.
Easy examples to solve complex problems.
Strong commitment to clearing student's doubts until he/she completely understands it.
Friendly and compassionate teaching so that a student can express better.
I teach because I love sharing knowledge more than anything else in the world. In the end, Quoting this stirring quote(one of my favorites) by Swami Vivekananda that I follow :
Take up one idea. Make that one idea your life - think of it, dream of it, live on that idea. Let the brain, muscles, nerves, every part of your body, be full of that idea, and just leave every other idea alone. This is the way to success.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
List major external and internal customer groups of apple Explain the value exchange for each customer group listed of apple
-
Use Table 9.4 to calculate an approximate enthalpy change for (a) The reaction of H 2 and C 2 H 2 to form 1 mol C 2 H 6 . (b) The reaction of molecular hydrogen and molecular nitrogen to form 1 mol...
-
Using Table 9.4, calculate an approximate enthalpy change for (a) The reaction of molecular hydrogen (H 2 ) and molecular oxygen (O 2 ) in the gas phase to produce 2 mol water vapor. (b) The reaction...
-
Show the parse trees for the two parses that the grammar assigns for sentence S1. S1: the train station bus rumbles [3 marks] (b) Give an algorithm for a bottom-up passive chart parser without...
-
Calculate the radius of a vanadium atom, given that V has a BCC crystal structure, a density of 5.96 g/cm 3 , and an atomic weight of 50.9 g/mol.
-
If H = yax xay A/m on plane z = 0, (a) Determine the current density and (b) Verify Ampere's law by taking the circulation of H around the edge of the rectangle Z = 0, 0 < x < 3, 1 < y < 4.
-
What does Herzbergs two-factor theory of motivation say about human motivation? How has it influenced current management practice? (L01)
-
Drab Corporation just obtained exclusive rights to a new revolutionary fertilizer that is sure to be an instant success in the gardening industry. Unfortunately, Drab does not control the capital to...
-
A corporation has Current Assets of $61, Property, Plant and Equipment of $698, Current Liabilities of $150, and Long Term Liabilities of $483. What is its Debt to Assets Ratio? Select one: a. 19.8%...
-
The equation for the combustion of gaseous methanol is Using the bond dissociation enthalpies, estimate the enthalpy change for this reaction. 2CH3OH(g) + 302(g) 2CO(g) + 4HO(g)
-
Use the octet rule to predict the element (E) from the second period that would be the central atom in the following ions. (a) EF (b) EF+
-
Look at the following pseudocode class definitions: Given these class definitions, what will the following pseudocode display? Class Plant Public Module message () Display "I'm a plant. End Module...
-
the assessment include developing gantt chart, work breakdown structure and and all task 3 are related to its respective task 2. all the instructions are given in the assignment itself. Assessment...
-
Mens heights are normally distributed with mean 68.6in. and standard deviation 2.8in. Air Force Pilots The U.S. Air Force required that pilots have heights between 64 in. and 77 in. Find the...
-
Swain Athletic Gear (SAG) operates six retail outlets in a large Midwest city. One is in the center of the city on Cornwall Street and the others are scattered around the perimeter of the city....
-
ACC1810 - PRINCIPLES OF FINANCIAL ACCOUNTING Project 11: Chapter 11 - Stockholders' Equity Part B: Financial Statements The accounts of Rehearsal Corporation are listed along with their adjusted...
-
Match the term to the description. Outcome evaluation Focuses on the accomplishments and impact of a service, program, or policy and its effectiveness in attaining its outcomes set prior to...
-
When pure reduced cytochrome c is added to carefully prepared mitochondria along with ADP, Pi , antimycin A, and oxygen, the cytochrome c becomes oxidized, and ATP is formed, with a P/O ratio...
-
Suppose that a business sells 6-month subscriptions to its monthly magazine. On January 1, the company receives a total of $600 for 10 subscriptions. To record this transaction, the company debits...
-
The rods each have the same 25-mm diameter and 600-mm length. If they are made of A992 steel, determine the forces developed in each rod when the temperature increases by 50° C. -600 mm 60 /60...
-
Determine the resultant internal loadings on the cross section at point D. 1.25 kN/m 1.5 m '0.5 m' 0.5 m'0.5 m
-
Determine the resultant internal loadings at cross sections at points E and F on the assembly. 1.25 kN/m 1.5 m '0.5 m'0.5 m'0.5 m
-
Difference between Operating Leverage and Financial Leverage
-
bpmn diagram for misc purchases
-
You have $55,000. You put 15% of your money in a stock with an expected return of 10%, $38,000 in a stock with an expected return of 18%, and the rest in a stock with an expected return of 22%. What...

Study smarter with the SolutionInn App


