Using the solubility rules in Table 4.1 as a guide, predict whether an insoluble product forms when
Question:
Using the solubility rules in Table 4.1 as a guide, predict whether an insoluble product forms when each of the following pairs of solutions is mixed. Write the balanced chemical equation if a precipitation reaction does occur.
(a) Pb(NO3)2 and sodium carbonate
(b) Ammonium bromide and AgClO4
(c) Potassium hydroxide and copper(II) chloride
(d) Aammonium bromide and cobalt(II) sulfate
Strategy
Write the formulas of the new potential ionic compounds by making a table of cations and anions and their combinations. Use the solubility rules to determine whether any of the combinations are insoluble.
Table 4.1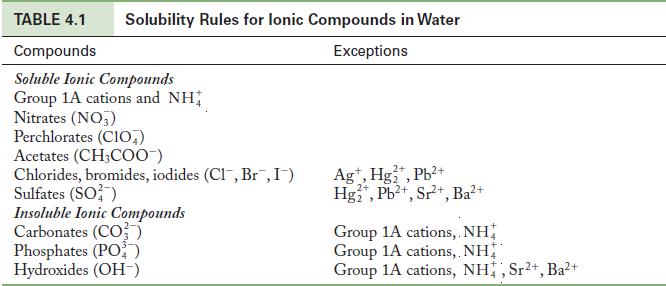
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





