Using the thermochemical equations in Exercise 5.67 as needed and in addition Exercise 5.67 Using the following
Question:
Using the thermochemical equations in Exercise 5.67 as needed and in addition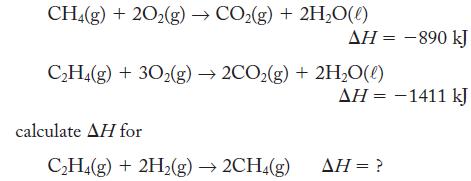
Exercise 5.67
Using the following thermochemical equations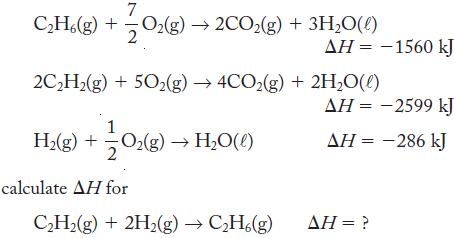
Transcribed Image Text:
CH4(g) + 20₂(g) → CO₂(g) + 2H₂O(l) C₂H4(g) + 30₂(g) →2CO₂(g) + 2H₂O(l) calculate AH for ΔΗ = -890 kJ C₂H4(g) + 2H₂(g) → 2CH4(g) AH = -1411 kJ AH = ?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (2 reviews)
To calculate AH for the reaction C2H4g2H2g 2CH4g we need to use Hesss Law which states that the change in enthalpy for a reaction is the same whether ...View the full answer

Answered By

Hillary Waliaulah
As a tutor, I am that experienced with over 5 years. With this, I am capable of handling a variety of subjects.
5.00+
17+ Reviews
30+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Which of the equations in Exercise 5 represent functions? Data from in Exercise 5 Which equations have a graph that is a vertical parabola? A horizontal parabola? A. y = -x + 20x + 80 C. x + 1 = (y...
-
In Gruber's (1970) study of n = 104 individuals (discussed in Problem 10), the relationship between blood pressure change (SBPSL) and relative weight (RW), controlling for initial blood pressure...
-
The number of counties, divisions, or parishes for each of the 50 states is given below. Use the data to construct a grouped frequency distribution with 6 classes, a histogram, a frequency polygon,...
-
The following items are dropped from an airplane. Rank them in order from lowest terminal speed to highest and justify your ranking. (a) Bowling ball (b) Beach ball (c) Spear or javelin (pointing...
-
For the conditions of Example 16.8, determine the effect on leaching time of particle size over the range of 0.5 mm to 50 mm.
-
On July 31, 2022, Sandhill Co. had a cash balance per books of $6,340.00. The statement from Dakota State Bank on that date showed a balance of $7,890.80. A comparison of the bank statement with the...
-
What percentage distinguishes a small stock dividend from a large stock dividend?
-
Jaeco Corporation asks you to review its December 31, 2014 inventory values and prepare the necessary adjustments to the books. The following information is given to you. 1. Jaeco uses the periodic...
-
Storico currently has 22,000 shares outstanding that sell for $46.23 per share. The company plans to issue a stock dividend of 15 percent. How many new shares will be issued?
-
Part of developing a long-term R&D strategy is to locate facilities in countries that are widely known to be competitive. Your company seeks to develop R&D facilities in Asia to counter recent...
-
Calculate H for the reaction Zn(s) + --0(g) ZnO(s) 2 given the equations AH = ? Zn(s) + 2HC1(aq) ZnCl (aq) + H(g) 2H(g) + O(g) 2HO(l) = -152.4 kJ ZnO(s) + 2HC1(aq) ZnCl(aq) + HO(0) AH = -90.2 kJ...
-
Using the following thermochemical equations 7 CH6(g) + O(g)2CO(g) + 3HO(l) 2CH(g) + 50(g) 4CO2(g) +2HO(l) H(g) + 0(g) HO(l) calculate AH for AH = -1560 kJ CH(g) + 2H(g) CH6(g) = -2599 kJ = -286...
-
What are individual and family brands? A national brand? A store brand? Appendix
-
do you agree wih this approach to dismantling the toxic culture? explain
-
Movies When randomly selecting a speaking character in a movie, the probability of getting a female is 0.331 (based on data from "Inequality in 1200 Popular Films," by Smith, et al., Annenberg...
-
Steve Reese is a well-known interior designer in Fort Worth, Texas. He wants to start his own business and convinces Rob O'Donnell, a local merchant, to contribute the capital to form a partnership....
-
Exercise 6-10A (Algo) Double-declining-balance and units-of-production depreciation: gain or loss on disposal LO 6-3, 6-4, 6-5 Exact Photo Service purchased a new color printer at the beginning of...
-
Independent Events Again assume that when randomly selecting a speaking character in a movie, the probability of getting a female is 0.331, as in Exercise 1. If we want to find the probability of 20...
-
How does checkpointing in ARIES differ from checkpointing as described in Section 23.1.4?
-
Assume a simple Keynesian depression economy with a multiplier of 4 and an initial equilibrium income of $3,000. Saving and investment equal $400, and assume full employment income is $4,000. a. What...
-
A weak acid has a dissociation constant of K a = 2.50 10 2 . a. Calculate the degree of dissociation for a 0.093m solution of this acid using the DebyeHckel limiting law. b. Calculate the degree of...
-
Calculate the mean ionic activity of a 0.0350 m Na 3 PO 4 solution for which the mean activity coefficient is 0.685.
-
At 25C, the equilibrium constant for the dissociation of acetic acid, K a , is 1.75 10 5 . Using the DebyeHckel limiting law, calculate the degree of dissociation in 0.150 m and 1.50 m solutions...
-
On April 1, year 1, Mary borrowed $200,000 to refinance the original mortgage on her principal residence. Mary paid 3 points to reduce her interest rate from 6 percent to 5 percent. The loan is for a...
-
Give a numerical example of: A) Current liabilities. B) Long-term liabilities?
-
Question Wonder Works Pte Ltd ( ' WW ' ) produces ceramic hair curlers to sell to department stores. The production equipment costs WW $ 7 0 , 0 0 0 four years ago. Currently, the net book value...

Study smarter with the SolutionInn App


