Vitamin A is converted by the body to retinal, a compound that is critical to human sight.
Question:
Vitamin A is converted by the body to retinal, a compound that is critical to human sight. The skeleton structure of vitamin A follows. Write the Lewis structure. How many sp2 and sp3 hybridized carbon atoms are in vitamin A?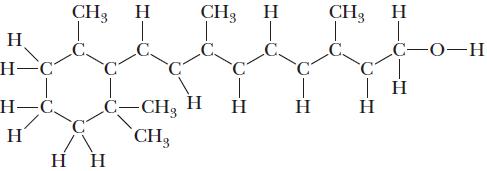
Transcribed Image Text:
H-C H-C, AR CH, CH, -CH3 CH3 CH, | H . T -0-H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
H C H CH H...View the full answer

Answered By

Nuzhat Nuzhat
I am experienced and passionate teacher dedicated to teaching . I love interacting with students, knowing their queries and solving them by providing core knowledge of the concept.
I focus on teaching the concepts in friendly but disciplined environment and welcome queries with patience.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Vitamin B6 is an organic compound whose deficiency in the human body can cause apathy, irritability, and an in-creased susceptibility to infections. Below is an incomplete Lewis structure, for...
-
The compound cyclohexane is an alkane in which six carbon atoms form a ring. The partial structural formula of the compound is as follows: (a) Complete the structural formula for cyclohexane. (b) Is...
-
The following are the financial statements of Swifty Corporation. Swifty Corporation Comparative Balance Sheets December 31 Assets 2019 2018 Cash $37,200 $19,700 Accounts receivable 33,000 18,400...
-
Write the shear and momentfunctions and draw shear and moment diagrams for the following frames: (a) Support A is a roller, B and C are fixed and support C is a pin.
-
Companies often try to keep accounting earnings growing at a relatively steady pace, thereby avoiding large swings in. earnings from period to period. They also try to me earnings targets. To do so...
-
In a forward punch in karate, the fist begins at rest at the waist and is brought rapidly forward until the arm is fully extended. The speed v(t) of the fist is given in figure for someone skilled in...
-
Examine the sample data below. a. Construct a stem-and-leaf plot to assess whether the data are from an approximately normal distribution. b. Compute s for the sample data. c. Find the values of QL...
-
Salari Industries, a small, family- run manufacturer, has adopted an ABC system. The following manufacturing activities, indirect manufacturing costs, and usage of cost drivers have been estimated...
-
Exercise 9 - 6 ( Algo ) Variable Overhead Variances [ LO 9 - 6 ] Logistics Solutions provides order fulfillment services for dot.com merchants. The company maintains warehouses that stock items...
-
A compound is analyzed and found to contain 54.53% carbon, 9.15% hydrogen, and 36.32% oxygen by mass. A mass spectrometry experiment shows that the molar mass is 44 g/mol. What is the molecular...
-
Write all important resonance structures for each of the following species, and use the VSEPR model to determine the bond angles around each central atom. Also indicate the hybrid orbitals on each...
-
In Exercises, recall that the slope of the tangent line to a graph is given by the derivative of the function. Find the slope of the tangent line to the graph of each equation at the given point. You...
-
When CH4(g) reacts with O2(g) to form CO2(g) and H2O(g), 192 kcal of energy are evolved for each mole of CH4(g) that reacts. Write a balanced equation for the reaction with an energy term in kcal as...
-
Ben Rogers, Judy Wilkinson, and Henry Walker were the partnership dentists. Ben Rogers became insolvent because of real estate investments. So, Judy Wilkinson and Henry Walker had to then pay the...
-
A random sample of 10 subjects have weights with a standard deviation of 10.8148 kg. What is the variance of their weights? Be sure to include the appropriate units with the result.
-
of stion 1. Harmonic Test II. Root Test III. Ratio Test Consider the series 8 = 72 Which one of the following tests can be used to determine whether it is convergent or divergent? IV. Integral Test...
-
Pharaoh company obtains $44,800 in cash by signing a 7%, 6 month, $44,800 note payable to First Bank on July 1. Pharoah's fiscal year ends on September 30. What information should be reported for the...
-
Chlorate (CO - 3 ), chlorite (CO - 2 ) , bromate (BrO - 3 ), and iodate (IO - 3 ) can be measured in drinking water at the 1-ppb level with 1% precision by selected reaction monitoring. Chlorate and...
-
Refer to the situation described inBE 18-13, but assume a 2-for-1 stock split instead of the 5% stock dividend. Prepare the journal entry to record the stock split if it is to be effected in the form...
-
The curved beam is subjected to a bending moment of M = 85 N · m as shown. Determine the stress at points A and B and show the stress on a volume element located at these points. M = 85 N-m...
-
A shaft is made of a polymer having a parabolic upper and lower cross section. If it resists a moment of M = 125 N · m, determine the maximum bending stress in the material (a) using the...
-
The composite beam consists of a wood core and two plates of steel. If the allowable bending stress for the wood is (Ï allow ) w = 20 MPa, and for the steel (Ï allow ) st = 130 MPa,...
-
A family has a $117,443, 25-year mortgage at 5.4% compounded monthly. (A) Find the monthly payment and the total interest paid. (B) Suppose the family decides to add an extra $100 to its mortgage...
-
Comparing the actual and planned cost of a consulting engagement completed by an engineering firm such as Allied Engineering.
-
What is the NPV of a project that costs $34,000 today and is expected to generate annual cash inflows of $11,000 for the next 7 years, followed by a final inflow of $14,000 in year 8. Cost of capital...

Study smarter with the SolutionInn App


