What are the products of each of the following acidbase reactions? Indicate the acid and its conjugate
Question:
What are the products of each of the following acidbase reactions? Indicate the acid and its conjugate base, and the base and its conjugate acid.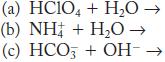
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
a Solution HClO4 H2O C1 H3O Acid Bas...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
What are the products of each of the following acidbase reactions? Indicate the acid and its conjugate base and the base and its conjugate acid. (a) HNO 3 + H 2 O (b) HSO 4 + H 2 O (c) H 3 O + + F
-
What are the products of each of the following acidbase reactions? Indicate the acid and its conjugate base and the base and its conjugate acid. (a) HClO 4 + H 2 O (b) NH 4 + + H 2 O (c) HCO 3 + OH
-
You continue to use the buffer that you prepared in Example 6G.1 but are worried about how adding sodium hydroxide to the buffer solution will affect the pH, which could upset your experiment....
-
A) Suppose you wanted to make a photoconductor that interacts in the visible, green light range (500-570 nm). Which of the following semiconductors would be your best choice, and why? Si, AIP, InAs,...
-
For a senior project, a student was given the assignment to design a heat exchanger that meets the following specifications: Like many real-world situations, the customer hasn't revealed, or doesn't...
-
Evelyn Kowalchuk, an eighty-eight-year-old widow, and her son, Peter, put their savings into accounts managed by Matthew Stroup. Later, they initiated an arbitration proceeding before the National...
-
2. Pop accounts for its investment in Son as a one-line consolidation. Prepare the consolidation workpaper entries related to this intercompany sale that are necessary to consolidate the financial...
-
The personnel director for a small manufacturing company has collected the data found in the file Dat9-19.xls on your data disk describing the salary (Y) earned by each machinist in the factory along...
-
Sabrina Company manufactures large leisure boats. The following schedule shows total fixed costs at various levels of boat production: Units Produced Total Fixed Costs 0100 $150,000 101400 250,000...
-
Write an equation to describe the proton transfer that occurs when each of these acids is added to water.
-
The following species react in aqueous solution. Predict the products, identify the acids and bases (and their conjugate species), and show the proton transfer in the acidbase reactions. (a) Ammonia...
-
What is forfaiting? How does it work? Why did it arise?
-
Use the information below to answer the next question. Below are different graphs that could represent the magnitude of an Electric Field from a source. Teza E Distance E 4 Tza E Taza 2 Distance 5 3...
-
Factor out the GCF: 36c5 +54c8
-
Demonstrate that a circle with a radius of r has a circumference of 2 pi ( r ) . HINT: Begin by examining the equation for the upper semicircle, utilize the arc length formula, and then double the...
-
Graph the function f(x) = 3.x - 7.
-
Vine plc. produces a single product. The following information on inventory, purchases, and sales are available for the month of January 2018. DATE TRANSACTION NUMBER OF UNITS UNIT COST...
-
Federal regulations set an upper limit of 50 parts per million (ppm) of NH3 in the air in a work environment [that is, 50 molecules of NH3(g) for every million molecules in the air]. Air from a...
-
In a nonmagnetic medium, E = 50 cos (10 9 t 8x) a y + 40 sin (10 9 t 8x) a z V/m find the dielectric constant r and the corresponding H.
-
Why was electromagnetic induction discovered much earlier than its converse, the production of a magnetic field by a changing electric field?
-
Why are light waves able to travel through a vacuum whereas sound waves cannot?
-
A double star consists of two nearby stars that revolve around their center of mass. How can an astronomer recognize a double star from the characteristic frequencies of the light that reaches him...
-
Deacon Company is a merchandising company that is preparing a budget for the three - month period ended June 3 0 th . The following information is available Deacon Company Balance Sheet March 3 1...
-
Mango Company applies overhead based on direct labor costs. For the current year, Mango Company estimated total overhead costs to be $460,000, and direct labor costs to be $230,000. Actual overhead...
-
Which of the following do we expect to be the horizon growth rate for a company (long term growth rate- say 30-50 years)? A) Inflation B) Industry Average C) Zero D) Market Beta

Study smarter with the SolutionInn App


