What is H rxn for reaction of iron(III) oxide and carbon monoxide to give iron metal and
Question:
What is ΔHrxn for reaction of iron(III) oxide and carbon monoxide to give iron metal and carbon dioxide gas? Use the following reactions: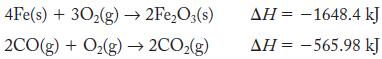
Transcribed Image Text:
4Fe(s) + 30₂(g) → 2Fe₂O3(s) 2CO(g) + O₂(g) → 2CO₂(g) AH-1648.4 kJ ΔΗ = -565.98 kJ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
To determine Hrxn for the reaction of ironIII oxide and carbon monoxide to give iron metal and carbo...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Iron(III) oxide reacts with carbon monoxide to give iron metal and carbon dioxide. If you begin the reaction with 24.0 g of iron(III) oxide and 34.0 g of carbon monoxide, what is the theoretical...
-
Solid oxide fuel cells (SOFC) have been proposed as an alternative energy technology for use in large stationary power applications (1 to 10MWof electrical power). These devices have an ion...
-
Blast furnaces extract pure iron from the iron(III) oxide in iron ore in a two step sequence. In the first step, carbon and oxygen react to form carbon monoxide: 2C (s) + O 2 (g) 2CO (g) In the...
-
How to respond to this response The effective utilization of data is integral to enhancing efficiency and making informed decisions?
-
An MSMPR-type crystallizer is to be designed to produce 2,000 lb/h of crystals of the heptahydrate of magnesium sulfate with a predominant crystal size of 35 mesh. The magma will be 15 vol% crystals....
-
Question 1 Fresh Air Products manufactures and sells a variety of camping products. Recently the company opened a new plant to manufacture a deluxe portable cooking unit. Cost and sales data for the...
-
Can a domestic company hedge the forecasted net income of a foreign subsidiary?
-
Employees at your company disagree about the accounting for sales returns. The sales manager believes that granting more generous return provisions and allowing customers to order items on a bill and...
-
Critique this statement: NPV is a better measure of project profitability than IRR because NPV leads to better capital investment decisions.
-
Ozone gas (O 3 , solute A) dissolved in high-purity water is commonly used in wet cleaning processes associated with semiconductor device fabrication. It is desired to produce a liquid water stream...
-
Ammonium nitrate, a common fertilizer, has been used by terrorists to construct car bombs. The products of the explosion of ammonium nitrate are nitrogen gas, oxygen gas, and water vapor. or ammonium...
-
A typical waterbed measures 84 in. 60 in. 9 in. How many kilocalories are required to heat the water in the waterbed from 55 F (cold water from the faucet) to 85 F, the operating temperature of the...
-
What kinds of portable IT help employees work more efficiently and effectively? What may interfere with productivity?
-
GATE 2024-EE Question
-
GATE 2024-EE Question
-
GATE 2024-EE Question
-
What is Netduino?
-
What is Appolonius theorem?
-
Calculate the internal rate of return on the following set of cash flows: t0: ............................-1,000 t1: ............................100 t2: ............................900 t3:...
-
What is your assessment of the negotiations process, given what you have studied? What are your recommendations for Mr. Reed? You must justify your conclusions
-
In the description of Figure 9.24b, the following sentence appears: At the point when the L 2 phase disappears, the temperature increases beyond 94°C and the vapor composition changes along the i...
-
Explain why chemists doing quantitative work using liquid solutions prefer to express concentration in terms of molality rather than molarity.
-
Explain the usefulness of a tie line on a PZ phase diagram such as that of Figure 9.4. Figure 9.4 100 Liquid 80 60 Liquid + vapor 40 Vapor 20 0.2 0.4 0.6 0.8 Zbenzene Pressure/Torr
-
The plant asset and accumulated depreciation accounts of Pell Corporation had the following balances at December 3 1 , 2 0 2 0 : Transactions during 2 0 2 1 were as follows: a . On January 2 , 2 0 2...
-
All else equal, a company's P/E ratio will ____________ when the discount rate ___________. rise, rise fall, rise fall, falls cannot be determined All else equal, a company's P/E ratio will _____...
-
The cost of partially completed goods at the end of the period would be Ending work in process inventory Cost of goods sold Beginning finished goods inventory Beginning work in process inventory

Study smarter with the SolutionInn App


