What is the wavelength of light, in nanometers, required to raise an electron in the O 7+
Question:
What is the wavelength of light, in nanometers, required to raise an electron in the O7+ ion from the n = 1 shell to the n = 2 shell?
Strategy
Use Equation 7.8 and the knowledge that a photon must have the same energy as the difference in energies of two quantized energy levels. Note that for an oxygen atom, Z = 8.
Equation 7.8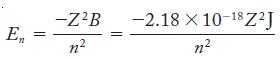
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





