Which of the following isotopes lie(s) within the band of nuclear stability shown in Figure 21.1 ?
Question:
Which of the following isotopes lie(s) within the band of nuclear stability shown in Figure 21.1 ?
Figure 21.1 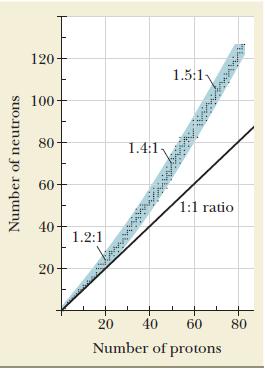
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
a 160 is often a stable isotope b C1 The isotope 37 Cl is typ...View the full answer

Answered By

Nyron Beeput
I am an active educator and professional tutor with substantial experience in Biology and General Science. The past two years I have been tutoring online intensively with high school and college students. I have been teaching for four years and this experience has helped me to hone skills such as patience, dedication and flexibility. I work at the pace of my students and ensure that they understand.
My method of using real life examples that my students can relate to has helped them grasp concepts more readily. I also help students learn how to apply their knowledge and they appreciate that very much.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
What are the activity coefcients for iron(III) and sulfate in an aqueous solution of 10 mM sulfuric acid, 5 mM calcium sulfate, and 3 mM iron(III) chloride?
-
You invest 50% of your portfolio in the market portfolio and 50% in the risk-free asset. What is your portfolio's CAPM beta?
-
Which of the following isotopes lie(s) within the band of nuclear stability shown in Figure 21.1 ? Figure 21.1
-
At the intersection of Texas Avenue and University Drive, a yellow subcompact car with mass 950 kg traveling east on University collides with a red pickup truck with mass 1900 kg that is traveling...
-
A simple perceptron cannot represent XOR (or, generally, the parity function of its inputs). Describe what happens to the weights of a four-input, step-function perceptron, and beginning with all...
-
Here are key financial data for House of Herring, Inc.: Earnings per share for 2015 ...................... $5.50 Number of shares outstanding ................. 40 million Target payout ratio...
-
Based on what characteristics can a market be segmented? Provide an example of each segmentation approach, showing how characteristics can be used to shape a marketing program.
-
Strategic analysis of operating income (continuation of 13-26). Refer to Exercise 13-26. 1. Calculate the operating income of Snyder Corporation in 2008 and 2009. 2. Calculate the growth,...
-
Current Attempt in Progress The merchandise inventory in Sandhill Co. was counted after the close of business on December 31, 2021, the company's year end. It was determined that the total cost of...
-
Write the balanced nuclear equation for each of the following nuclear decays. Show the mass and atomic numbers for each of the particles in the equation. (a) Alpha decay of bismuth-201 (b) Positron...
-
Calculate the neutron-to-proton ratio (to two decimal places) for each of the following stable isotopes.
-
Describe an interest rate cap.
-
Kelly Corporation received an advanced payment of \(\$ 30,000\) in 2018 from Rufus Company for consulting services. Kelly performed half of the consulting in 2018 and the remainder in 2019. Kelly...
-
Rosa Dominguez, the owner of Elegant Dining in San Jose, California, is pondering whether to buy electronic menu technology and tablets for her five-star restaurant. Prices for a typical four course...
-
Dura Corporation makes metal frames for several world brands of portable home generators. They sell the frames to a wide variety of portable generator manufacturers such as DeWalt, DuroMax, Generac,...
-
Rocker Industries (RI) produces recreational in-line skates (see Exhibit 14.36). Demand is seasonal, peaking in the summer months, with a smaller peak demand during December. For one of their more...
-
The BOM, current inventory, and lead time (in months) for the in-line skates in Rocker Industries (A) case is shown in Exhibit 14.37. Using the chase demand strategy, you developed in Rocker...
-
A random sample of 120 observations was selected from a binomial population, and 72 successes were observed. Do the data provide sufficient evidence to indicate that p is greater than .5? Use one of...
-
Use translations to graph f. f(x) = x-/2 +1
-
A rectangular channel must carry 2.0 m 3 /s of water from a water-cooled refrigeration condenser to a cooling pond. The available slope is 75 mm over a distance of 50 m. The maximum depth of flow is...
-
Calculate the depth of flow in a trapezoidal channel with a bottom width of 3 m and whose walls slope 40 with the horizontal. The channel is made of unfinished concrete and is laid on a 0.1-percent...
-
Calculate the depth of flow of water in a rectangular channel 10 ft wide, made of brick in cement mortar, for a discharge of 150 ft 3 /s. The slope is 0.1 percent.
-
why would an auditor want to complete dual-purpose tests? what procedure can be put into place to help prevent fraud? List 4 procedures.
-
Based on the following information, calculate sustainable growth rate for Groot, Inc.: Profit margin= 7.1% Total asset turnover = 1.90 Total debt ratio = .45 Payout ratio = 20% What is the ROA here?
-
Consider the following: a call option on a stock has strike price $100, premium of $5 and the current price of the underlying stock is $100. If you buy the call option today, what is your holding...

Study smarter with the SolutionInn App


