Write a balanced equation for each of these combustion reactions. (a) C4H10(g) + O(g) (b) C6H12O6(s)
Question:
Write a balanced equation for each of these combustion reactions.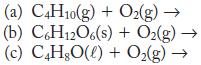
Transcribed Image Text:
(a) C4H10(g) + O₂(g) → (b) C6H12O6(s) + O₂(g) → (c) C4H8O(l) + O₂(g) →>>
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)
balanced chemical equations for each of these combustion reactions a C...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Write a balanced equation for each reaction. (a) (b) (c) (d) H SO, heat CH3 CH2CH-CH NaOC(CH3 3 Br Br Nal CHCH CH-CH acetone NaOH, heat CH3 CH CCH3 Br
-
Write a balanced equation for the combustion of benzoic acid, C6H5CO2H, to give CO2 and H2O. How many milligrams of CO2 and of H2O will be produced by the combustion of 4.635 mg of benzoic acid?
-
Write a balanced equation for each of the following reactions or reaction sequences. (a) The reaction catalyzed by PFK-2 (b) The conversion of 2 moles of oxaloacetate to glucose (c) The conversion of...
-
An interest payment of $650 in a 20 percent tax bracket would result in a tax savings of _____.
-
A transmission line is terminated by a load with admittance YL = (0.6 4 + j0.8)/Zo. Find the normalized input impedance at /6 from the load.
-
Question 1: In 2015, Mordica Co. issued 200,000 shares of $10 par value ordinary shares at $35 per share. In January, 2016, Mordica repurchased 15,000 shares at $30 per share. Assume these are the...
-
Household food consumption. The data in the table below were collected for a random sample of 26 households in Washington, D.C. An economist wants to relate household food consumption, y, to...
-
Mystic Inc. uses a job order costing system and applies overhead to jobs at a predetermined rate of $4.25 per direct labor dollar. During April 2010, the company spent $29,600 on direct material and...
-
A farmer sows a certain crop. It costs $360,000 to buy the seed, prepare the ground, and sow the crop. In one years, time it will cost $120,000 to harvest the crop. If the crop will be worth...
-
16.129 La barra AB de 2 kg y la barra BC de 3 kg se conectan como muestra a un disco que se pone a girar en un plano vertical a una velocidad ang lar constante de 6 rad/s en el sentido de las...
-
Write a balanced equation for the combustion (in excess oxygen) of each of the following compounds. (a) C 6 H 12 (b) C 4 H 8 (c) C 2 H 4 O (d) C 4 H 6 O 2
-
Write a balanced equation for the reaction of (a) Mg(OH) 2 and HF. (b) Sodium hydroxide and HCl. (c) H 2 SO 4 and strontium hydroxide.
-
Lilia is going to be subject to the AMT in 2019. She owns an investment building and is considering disposing of it and investing in other realty. Based on an appraisal of the buildings value, the...
-
Albert is in third grade and has documented impulsivity issues in class. Develop a plan to teach Albert how to answer questions in class appropriately. He will currently shout out answers and if the...
-
What type of atmosphere is generated in the zara locations? How do the stores draw in their customers? Is there any atmospherics that would make you stay in the stores? Is it enjoyable inside, does...
-
You've been asked to create a machine learning service that helps people choose what concert to attend on a particular date based on the type of music they prefer, who is singing, and where the event...
-
What are the lessons (human resource, marketing, services, location, pricing, etc.) that Disney learned from its previous international ventures (Japan, EDL, HK)? What were some of the mistakes and...
-
17.C. a. A person asks you to convert a given point (x,y) into polar coordinates (r, 0). Explain how this might be an ambiguous question (i.e., is further information needed?). b. There is only 1 out...
-
Although the stereochemistry of Eq. 27.39 cannot be determined from the reaction, what stereochemistry is expected from orbital symmetry considerations?
-
Why is a help desk and production support critical to system implementations? Discuss its interrelationship with the problem management and reporting system.
-
Calculate O2 mixture (298 15 K, 1 bar) , for oxygen in air, assuming that the mole fraction of O 2 in air is 0.210. Use the conventional molar Gibbs energy defined in Section 6.17.
-
Predict the product of the following reaction. Br NaOCH3, heat -NO2
-
What is the relationship between the K P for the two reactions 3/2H 2 (g) + 1/2N 2 (g) NH 3 (g) and 3H 2 (g) + N 2 (g) 2NH 3 (g)?
-
Given that rJ = 6.3%, rRF = 4.1%, and rM = 9.4%, determine the beta coefficient for Stock J that is consistent with equilibrium.
-
Simon Companys year-end balance sheets follow. At December 31 2017 2016 2015 Assets Cash $ 33,019 $ 37,839 $ 38,623 Accounts receivable, net 93,822 65,556 54,152 Merchandise inventory 117,963 89,253...
-
PLEASE REFER TO THE 2018 ANNUAL REPORT OF STARBUKS FOR THE YEAR FISCAL YR 2018, ENDING SEPTEMBER 30, 2018. Refer to the management discussion & analysis section and write a one page summary...

Study smarter with the SolutionInn App


