Predict the product of the following reaction. Br NaOCH3, heat -NO2
Question:
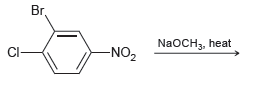
Transcribed Image Text:
Br NaOCH3, heat -NO2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 88% (9 reviews)
Br CI ...View the full answer

Answered By

Akshay Singla
as a qualified engineering expert i am able to offer you my extensive knowledge with real solutions in regards to planning and practices in this field. i am able to assist you from the beginning of your projects, quizzes, exams, reports, etc. i provide detailed and accurate solutions.
i have solved many difficult problems and their results are extremely good and satisfactory.
i am an expert who can provide assistance in task of all topics from basic level to advance research level. i am working as a part time lecturer at university level in renowned institute. i usually design the coursework in my specified topics. i have an experience of more than 5 years in research.
i have been awarded with the state awards in doing research in the fields of science and technology.
recently i have built the prototype of a plane which is carefully made after analyzing all the laws and principles involved in flying and its function.
1. bachelor of technology in mechanical engineering from indian institute of technology (iit)
2. award of excellence in completing course in autocad, engineering drawing, report writing, etc
4.70+
48+ Reviews
56+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Predict the product of the following Diels-Alderreaction: - C=C
-
Predict the product of the following Diels?Alder reaction: ?
-
Predict the product of the following pericyclic reaction. Is this [5, 5] shift a suprafacial or an antarafacialprocess? [5,5] CH eat
-
Use a calculator to obtain solutions correct to the nearest hundredth in Problems 4954. x + 4 = 32x
-
Why do so many entrepreneurs run into trouble when they buy an existing business? Outline the steps involved in the right way to buy a business. (LO 3: Explain the four steps in the search stage for...
-
In chemistry, the pH value of a solution is a measure of its acidity. The pH value is defined by pH = log(H + ), where H + is the hydrogen-ion concentration. If the pH of a sample of rainwater is...
-
P 20-8 Debt service fund journal entries The Town of Lilehammar has $3,000,000 of 6 percent bonds outstanding. Interest on the general obligation, general government indebtedness is payable...
-
Assume Earths orbit to be circular and that the Suns mass suddenly decreases by half. What orbit does Earth then have? Will Earth escape the solar system?
-
Question 2 10 pts Using the cost data for Project X and Project Y below, conduct a Present Worth Analysis to calculate the Present Worth of Project X at time over LCM years of the life of both X and...
-
Given a database of the results of an election, find the number of seats won by each party. There are some rules to going about this: There are many constituencies in a state and many candidates who...
-
Calculate O2 mixture (298 15 K, 1 bar) , for oxygen in air, assuming that the mole fraction of O 2 in air is 0.210. Use the conventional molar Gibbs energy defined in Section 6.17.
-
What is the relationship between the K P for the two reactions 3/2H 2 (g) + 1/2N 2 (g) NH 3 (g) and 3H 2 (g) + N 2 (g) 2NH 3 (g)?
-
Where is most of the glacial ice located today?
-
A production Edgeworth Box, with origins indicated for the inputs of capital, K , and labor, L , into production of goods X and Y .Eight isoquants are shown, reflecting standard...
-
For 2014, Nichols, Inc., had sales of 150,000 units and production of 200,000 units. Other information for the year included: Direct manufacturing labor 187,500 Variable manufacturing overhead...
-
reading the following statement and decide whether you agree or disagree with the statement: "The free market system is the best economic system since it is the most efficient and solves basic...
-
find the net presbf value of the project ? present value index? Net present value A project has estimated annual net cash flows of $11,250 for 10 years and is estimated to cost $42,500. Assume a...
-
Calculate the ICER for the new treatment, without adjusting for the health utility index. Assuming the $50K benchmark*, as a clinical decision maker or health policy advisor, would you recommend...
-
The pH scale is used to measure the acidity or alkalinity of a solution. The scale ranges from 0 to 14. A neutral solution, such as pure water, has a pH of 7. An acid solution has a pH less than 7...
-
In each of the following independent cases, document the system using whatever technique(s) your instructor specifies. a. Dreambox Creations (www.dreamboxcreations.com/) in Diamond Bar, California,...
-
Tell why each of the following structures is not consistent with the spectroscopic data in Study Problem 13.8. Problem 13.8. Specify whether the labeled protons in each of the following structures...
-
How would you distinguish among the compounds within each of the following sets using their NMR spectra? Explain carefully and explicitly what features of the NMR spectrum you would use. (a)...
-
How would you distinguish among the compounds within each of the following sets using their NMR spectra? Explain carefully and explicitly what features of the NMR spectrum you would use. (a)...
-
Hite corporation intends to issue $160,000 of 5% convertible bonds with a conversion price of $40 per share. The company has 40,000 shares of common stock outstanding and expects to earn $600,000...
-
Your portfolio has a beta of 1.17, a standard deviation of 14.3 percent, and an expected return of 12.5 percent. The market return is 11.3 percent and the risk-free rate is 3.1 percent. What is the...
-
Slow Roll Drum Co. is evaluating the extension of credit to a new group of customers. Although these customers will provide $198,000 in additional credit sales, 13 percent are likely to be...

Study smarter with the SolutionInn App


