Predict the product of the following Diels?Alder reaction: ?
Question:
Predict the product of the following Diels?Alder reaction:
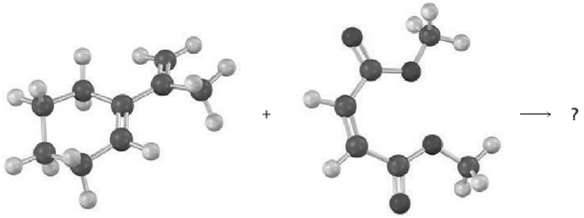
Transcribed Image Text:
?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 58% (17 reviews)
Rotation of the diene to th...View the full answer

Answered By

Ishrat Khan
Previously, I have worked as an accounting scholar at acemyhomework, and have been tutoring busines students in various subjects, mostly accounting. More specifically I'm very knowledgeable in accounting subjects for college and university level. I have done master in commerce specialising in accounting and finance as well as other business subjects.
5.00+
140+ Reviews
437+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Predict the product of the following reactions. (a) (b) (c) (d) (e) (f) (g) (h) (i) HBr (excess), OH (1) NaH OH Br2 (excess) H20 H3C OH excess QH (1) NaH,Br (2) heat HNO3, H2SO4 NaNH2, NHa (1) NaOH...
-
Predict the product of the following reaction. O (1) BrMg (2) H2O MgBr (1 equiv.)
-
Predict the product of the reaction if CH3-S-CH2CH2CH2CH2-Br is heated in a polar organic solvent, such as methanol. Similarly, what would be the product for hexyl bromide in methanol? (In hexyl...
-
Dixie Irwin is the department manager for Religious Books, a manufacturer of religious books that are sold through Internet companies. Irwins bonus is based on reducing production costs. Irwin has...
-
Why is infrastructure essential to economic development?
-
A latte (which means milk in Italian) is a coffee drink made with milk and often topped with milk foam. According to the Starbucks menu, its Grande lattes contained 16 fl. Oz. A group of heated...
-
Define informal communication.
-
The following financial data were reported by 3M Company for 2008 and 2009 (dollars in millions). Instructions(a) Calculate the current ratio and working capital for 3M for 2008 and 2009.(b) Suppose...
-
part c and d please 11:44 1 .. 5G 4 Disposal of Fixed Asset Equipment acquired on January 6 at a cost of $455,400 has an estimated useful life of 10 years and an estimated residual value of $59,400....
-
Assume that Valley Forge Hospital has only the following three payer groups: The hospitals fixed costs are $38 million. a. What is the hospitals net income? b. Assume that half of the 100,000 covered...
-
Which of the following dienes have an s-cis conformation, and which have an s-trans conformation? Of the s-trans dienes, which can readily rotate tos-cis? (a) (c) (b)
-
Draw a segment of the polymer that might be prepared from 2-phenyl-1, 3-butadiene.
-
Find all values and graph some of them in the complex plane. ln (-7)
-
Jimmy Joe-Bob Hicky is the district commander for the mostly-rural Spud Valley highway patrol district in western Idaho. Hes attempting to assign highway patrol cars to different road segments in his...
-
Its important to have a holistic view of all the businesses combined and ensure that the desired levels of risk management and return generation are being pursued. Agree or disagree
-
(3pts each) During a trip to a casino, Adam Horovitz plays his favorite casino game 10 times. Each time he plays, he has a 41% chance of winning. Assume plays of the game are independent. a. What is...
-
An epidemiologist plans to conduct a survey to estimate the percentage of women who give birth. How many women must be surveyed in order to be 95% confident that the estimated percentage is in error...
-
Statement of financial position as at 31 December 2014 ASSETS Non-current assets Property, plant and equipment Delivery van at cost 12,000 Depreciation (2,500) 9,500 Current assets Inventories...
-
Describe the process of conducting quantitative data analysis
-
A 20-cm-square vertical plate is heated to a temperature of 30oC and submerged in glycerin at 10oC. Calculate the heat lost from both sides of the plate.
-
What is the effect on vapor pressure of a solution with particularly strong solutesolvent interactions? With particularly weak solutesolvent interactions?
-
(a) Use the polygon rule to draw an energy diagram (as in Figures 16-5 and 16-7) for the MOs of a planar cyclooctatetraenyl system. In Figure 16.5 In Figure 16.7 (b) Fill in the eight pi electrons...
-
Step 2 of the iodination of benzene shows water acting as a base and removing a proton from the sigma complex. We did not consider the possibility of water acting as a nucleophile and attacking the...
-
In an aqueous solution containing sodium bicarbonate, aniline reacts quickly with bromine to give 2, 4, 6-tribromoaniline. Nitration of aniline requires very strong conditions, however, and the...
-
The balance of Factory Overhead at the end of the year is disposed of by transferring it to the _____. a.non-operating expense account b.accounts payable account c.cost of goods sold account...
-
Metlock Co. reports the following information for 2017: sales revenue $774,900, cost of goods sold $529,600, operating expenses $88,500, and an unrealized holding loss on available-for-sale...
-
Fiscal Year Ending: 12/31/2019 12/31/2018 12/31/2017 12/31/2016 Revenue Growth and Common Size Values (% of Revenue) FY 2019 FY 2018 FY 2017 FY 2016 FY 19 FY 18 FY 17 FY 16 Total Revenue $280,522,000...

Study smarter with the SolutionInn App


