Predict the product of the following pericyclic reaction. Is this [5, 5] shift a suprafacial or an
Question:
Predict the product of the following pericyclic reaction. Is this [5, 5] shift a suprafacial or an antarafacialprocess?
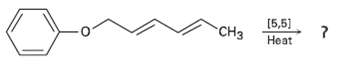
Transcribed Image Text:
[5,5] CHз Нeat
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 76% (13 reviews)
3 2 4 5 CH3 55 shift heat 2 3 CH3 ...View the full answer

Answered By

Asd fgh
sadasmdna,smdna,smdna,msdn,masdn,masnd,masnd,m asd.as,dmas,dma.,sd as.dmas.,dma.,s ma.,sdm.,as mda.,smd.,asmd.,asmd.,asmd.,asm
5.00+
1+ Reviews
15+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Predict the product of the following reactions. (a) (b) (c) (d) (e) (f) (g) (h) (i) HBr (excess), OH (1) NaH OH Br2 (excess) H20 H3C OH excess QH (1) NaH,Br (2) heat HNO3, H2SO4 NaNH2, NHa (1) NaOH...
-
Predict the product of the following reaction. O (1) BrMg (2) H2O MgBr (1 equiv.)
-
Predict the product of the following reaction:
-
Using the aggregate expenditures table below, answer the questions that follow. a. Compute the APC when income equals $2,300 and the APS when income equals $2,800. b. Compute the MPC and MPS. c. What...
-
Why do you believe the regulators approved the deal despite the large increase in industry concentration and their awareness that historically increases in concentration would likely result in a...
-
Marla is married, is paid $2,565 semimonthly, and claims four withholding allowances. What is the amount of federal income tax withheld on Marlas gross wages for the semimonthly period? Use the...
-
Explain why the true positive/false positive/true negative/false negative usage is not applicable to classification models with trinary targets.
-
Perney Company uses both standards and budgets. For the year, estimated production of Product X is 500,000 units. Total estimated cost for materials and labor are $1,200,000 and $1,600,000. Compute...
-
Neubert Corporation manufactures and sells a single product. The company uses units as the measure of activity in its budgets and performance reports. During December, the company budgeted for 5,300...
-
Develop a general-purpose computer application (using EES or other software) that employs the affinity laws to design a new turbine (B) that is dynamically similar to a given turbine (A). The inputs...
-
The following thermal rearrangement involves two pericyclic reactions in sequence. Identify them, and propose a mechanism to account for the observedresult. 275 "C CD2 - -D H2C CD2
-
Ring-opening of the trans-Cyclobutene isomer shown takes place at much lower temperature than a similar ring-opening of the cis-Cyclobutene isomer. Explain the temperature effect, and identify the...
-
In their 2017 financial statements, five international fashion retailers that is, Burberry, Esprit, French Connection, Inditex, and Next explicitly discussed their key accounting policies. The...
-
A 447 gram cart (mA) slides along a very smooth track and collides with a stationary 475 gram cart (mB). A motion detector records the velocity of cart A, as shown in Figures 1 and 2. A force probe...
-
M8 Homework i Saved 1 Mayfair Company completed the following transactions and uses a perpetual inventory system. Help Save & Exit Submit Check my work 10 points eBook Print References June 4 Sold...
-
Free Response Table Problem x -6 -80 -4 -3 f(x) 1.948 1 0 -2 -2.005 -798 undefined -2 -1.995 0 1 1.995 2 2.005 6 80 802 4 3.333 3.001 undefined 2.998 2.5 2.048 23. The table above represents values...
-
5. [-/0 Points] DETAILS OSPRECALC1 2.2.106. Use algebra to find the point at which the line f(x) = -x 258 -X+ intersects the line h(x) = x+ 91 + 25 10 (x, y) = Additional Materiale MY N
-
What does the graph tells? from your own understanding. CoursHeroTranscribedText 136 DIVIDED ATTENTION COUNTED TIME BACKWARDS 134 1 2 3 130 136 UNDIVIDED ATTENTION COUNTED TIME BACKWARDS 134 5 132...
-
Lead a final discussion in which participants share their findings and make summary comments about voice and its role in delivering effective service.
-
9.Consider the reaction 3NO2(g)+H2O=2HNO3(aq)+NO(g) where Delta H=-137 kJ.How many kilojoules are released when 92.3g of NO2 reacts?
-
During the winter of 2021, Robinhood Securities, a brokerage firm popular with retail investors, temporarily restricted trading certain highly volatile securities, including shares of GameStop. The...
-
Would you expect the 2-octanol formed by SN2 hydrolysis of (-)- 2-bromooctane to be optically active? If so, what will be its absolute configuration and sign of rotation? What about the 2-octanol...
-
Identify the compound in each of the following pairs that reacts with sodium iodide in acetone at the faster rate: (a) 1-Bromopentane or 3-bromopentane (b) 2-Chloropentane or 2-fluoropentane (c)...
-
Sodium nitrite (NaNO2) reacted with 2-iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula C8H17NO2 in a combined yield of 88%. Suggest reasonable structures...
-
What is Apple Companys strategy for success in the marketplace? Does the company rely primarily on customer intimacy, operational excellence, or product leadership? What evidence supports your...
-
Exercise 1 1 - 7 ( Algo ) Net present value and unequal cash flows LO P 3 Gomez is considering a $ 2 1 0 , 0 0 0 investment with the following net cash flows. Gomez requires a 1 2 % return on its...
-
a Campbell Inc. produces and sells outdoor equipment. On July 1, 2011. Campbell issued $40,000,000 a 10-year, 10% bonds at a market (effective) interest rate of 9%, receiving Cash of 548,601,480....

Study smarter with the SolutionInn App


