Ring-opening of the trans-Cyclobutene isomer shown takes place at much lower temperature than a similar ring-opening of
Question:
Ring-opening of the trans-Cyclobutene isomer shown takes place at much lower temperature than a similar ring-opening of the cis-Cyclobutene isomer. Explain the temperature effect, and identify the stereochemistry of each reaction as either conrotatory ordisrotatory.
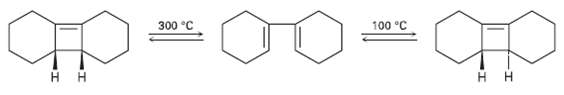
Transcribed Image Text:
100 °C 300 °C
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 36% (11 reviews)
HH HH conrotatory conrotatory Ring opening of the tra...View the full answer

Answered By

Aysha Ali
my name is ayesha ali. i have done my matriculation in science topics with a+ . then i got admission in the field of computer science and technology in punjab college, lahore. i have passed my final examination of college with a+ also. after that, i got admission in the biggest university of pakistan which is university of the punjab. i am studying business and information technology in my university. i always stand first in my class. i am very brilliant client. my experts always appreciate my work. my projects are very popular in my university because i always complete my work with extreme devotion. i have a great knowledge about all major science topics. science topics always remain my favorite topics. i am also a home expert. i teach many clients at my home ranging from pre-school level to university level. my clients always show excellent result. i am expert in writing essays, reports, speeches, researches and all type of projects. i also have a vast knowledge about business, marketing, cost accounting and finance. i am also expert in making presentations on powerpoint and microsoft word. if you need any sort of help in any topic, please dont hesitate to consult with me. i will provide you the best work at a very reasonable price. i am quality oriented and i have 5 year experience in the following field.
matriculation in science topics; inter in computer science; bachelors in business and information technology
_embed src=http://www.clocklink.com/clocks/0018-orange.swf?timezone=usa_albany& width=200 height=200 wmode=transparent type=application/x-shockwave-flash_
4.40+
11+ Reviews
14+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
A gaseous reaction takes place at constant volume and constant pressure in a cylinder shown here. Which of the following equations best describes the reaction? The initial temperature (T1) is twice...
-
A chemical reaction takes place in a container of cross-sectional area 50.0 cm/. As a result of the reaction, a piston is pushed out through 15 cm against an external pressure of 121 kPa. Calculate...
-
Explain why the reaction shown in eq. 7.19 occurs much more easily than the reaction (That is, why is it necessary to protonate the alcohol before ionization can occur?) (CH) C-OH(CHCHO
-
At the beginning of the year, Plummer's Sports Center bought three used fitness machines from Advantage, Inc. The machines immediately were overhauled, installed, and started operating. The machines...
-
Speculate as to why the share prices of American and US Airways increased sharply on the day that the agreement with the Justice Department had been reached? Why did the share prices of other major...
-
Would you expect nitrous acid to induce a higher frequency of Tyr Ser or Tyr Cys substitutions? Why?
-
Explain the notation used in the notation in this chapter, for the marginal totals and the grand total of the contingency tables.
-
Flower Company started doing business on January 1, 2013. For the year ended December 31, 2014, it reported $450,000 pre-tax book income on its income statement. Flower is subject to a 40% corporate...
-
Prepare an income statement for the year ending December 31, 2018 and a balance sheet at December 31, 2018. Assume semiannual compounding of the bond interest. Compute debt assets ratio and times...
-
Cullumber Industries collected $104,000 from customers in 2022. Of the amount collected, $24,300 was for services performed in 2021. In addition, Cullumber performed services worth $40,300 in 2022,...
-
Predict the product of the following pericyclic reaction. Is this [5, 5] shift a suprafacial or an antarafacialprocess? [5,5] CH eat
-
Photolysis of the cis-Cyclobutene isomer in Problem 30.25 yields cLc-cyclododecaen-7-yne, hut photolysis of the trans isomer yields trans-cyclododecaen-7-yne. Explain these results, and identify the...
-
Why is it important to establish standards for the use of terms such as eco?
-
(ii) State Wilkie's updating equation in respect of the force of inflation and explain carefully what each of the components of the equation represents. State also which type of time series process...
-
Compute the double integral D x y dA over the domain D indicated as 0 x 5, x y 2x + 3. (Use symbolic notation and fractions where needed.) f(x, y) A = D
-
4. (10 points) A researcher believes that length of time spent listening to classical music increases memory for previously learned material. She has 4 groups of 5 subjects listen to either 10 min.,...
-
We find a binary system consisting of a 1 solar mass star, still in its main sequence phase, and a white dwarf. Assume both stars formed at the same time and that they did not significantly influence...
-
The equity sections from Atticus Group's 2015 and 2016 year-end balance sheets follow. Stockholders Equity (December 31, 2015) Common stock $6 par value, 50,000 shares authorized, 35,000 shares...
-
Which type of formal communication do you think is most challengingupward, downward, or horizontal? Give your reasons.
-
How has the globalization of firms affected the diversity of their employees? Why has increased diversity put an additional burden on accounting systems?
-
Long-term U.S. Treasury bonds are considered to be very high-quality investments with virtually zero default risk. Is it reasonable to assume that the risk of settling U.S. Treasury bonds is also of...
-
Suggest a structure for the product of nucleophilic substitution obtained on solvolysis of tert-butyl bromide in methanol, and outline a reasonable mechanism for its formation.
-
Identify the compound in each of the following pairs that reacts at the faster rate in an SN1 reaction: (a) Cyclopentyl iodide or 1-methylcyclopentyl iodide (b) Cyclopentyl bromide or...
-
Use curved arrows to show how calcium carbide reacts with water to give acetylene.
-
Marie Forleo, a marketing trainer and host of MarieTV, presents the eight tips for genuine networking. Do you agree or disagree with her suggestions? Discuss how this information is useful to you and...
-
Identify all relevant costs or revenue that are applicable to production- constrained decisions 1. Contributions margin of product 2. Interference with other production 3. Contribution margin per...
-
Gammaro Compary manufactures wallets from fabric. In 2 0 1 9 , Gammaro made 2 , 1 5 0 , 0 0 0 wallets using 1 , 2 5 0 , 0 0 0 yards of fabric. In 2 0 1 9 , Gammaro has capacity to make 2 , 8 0 0 , 0...

Study smarter with the SolutionInn App


