Write all important resonance structures for each of the following species, and use the VSEPR model to
Question:
Write all important resonance structures for each of the following species, and use the VSEPR model to predict the bond angles around each central atom. Also indicate the hybrid orbitals on each central atom and whether the molecule is polar or nonpolar. Does each resonance structure use the same hybrid orbitals?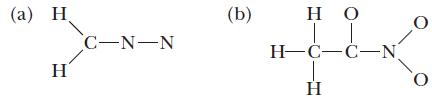
Transcribed Image Text:
(a) C-N-N (b) IT HC-C-N
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
Lets analyze the species given in the image step by step starting with compound a then moving on to compound b Compound a H2CNN 1 Resonance Structures For compound a we are looking at a diazomethane m...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Write all important resonance structures for each of the following species, and use the VSEPR model to determine the bond angles around each central atom. Also indicate the hybrid orbitals on each...
-
Write one important resonance structure for each of the following species, and use the VSEPR model to predict the bond angles around each central atom. Also indicate the hybrid orbitals on each...
-
Write the important resonance structures for each of the following: (a) (b) (c) (d) (e) (f) (g) (h) (i) (j) CH2 CH-Br NO2
-
Arginine, the most basic of the 20 common amino acids, contains a guanidino functional group in its side chain. Explain, using resonance structures to show how the protonated guanidino group...
-
Net Fixed Assets and Depreciation On the balance sheet, the net fixed assets (NFA) account is equal to the gross fixed assets (FA) account (which records the acquisition cost of fixed assets) minus...
-
You have just arrived in a new city and would like to see its sights. Each sight is located in a square and you have assigned each a beauty value. Each road to a square takes an amount of time to...
-
A part processed in a flexible manufacturing system (FMS) is routed through a set of operations, some of which are sequential and some of which are parallel. In addition, an FMS operation can be...
-
On January 1, 2015, Fisher Corporation paid $2,290,000 for 35 percent of the outstanding voting stock of Steel, Inc., and appropriately applies the equity method for its investment. Any excess of...
-
Edgehill, Inc. has 145,000 bonds outstanding. The bonds have a par value of $2,000, a coupon rate of 5.3 percent paid semiannually, and 10 years to maturity. The current YTM on the bonds is 4.9...
-
Ionization energies can be determined for molecules and atoms. Draw the molecular orbital diagrams for NO and CO, and predict which compound has the lower ionization energy.
-
More than 5 billion pounds of ethylene oxide, C 2 H 4 O, is produced annually. Ethylene oxide is used in the production of ethylene glycol, HOCH 2 CH 2 OH, the main component of antifreeze, and...
-
What other compiler options are available for your compiler and what do they do?
-
The following information appears in the records of Poco Corporation at year-end: a. Calculate the amount of retained earnings at year-end. b. If the amount of the retained earnings at the beginning...
-
For the following four unrelated situations, A through D, calculate the unknown amounts appearing in each column: A B D Beginning Assets... $38,000 $22,000 $38,000 ? Liabilities.. 22,000 15,000...
-
On December 31, John Bush completed his first year as a financial planner. The following data are available from his accounting records: a. Compute John's net income for the year just ended using the...
-
Statement of Stockholders' Equity and Balance Sheet The following is balance sheet information for Flush Janitorial Service, Inc., at the end of 2019 and 2018: Required a. Prepare a balance sheet as...
-
Petty Corporation started business on January 1, 2019. The following information was compiled by Petty's accountant on December 31, 2019: Required a. You have been asked to assist the accountant for...
-
Isotopic compounds are separated in Figure 22-15 by repeated passage through a pair of columns. Each cycle in the figure represents one pass through length L = 50 cm containing N theoretical plates....
-
Multiple Choice Questions: 1. The largest component of aggregate demand is? a. Government purchases. b. Net exports. c. Consumption. d. Investment. 2. A reduction in personal income taxes, other...
-
If the allowable bending stress is Ï allow = 6 MPa, determine the minimum dimension d of the beams crosssectional area to the nearest mm. 125 mm 25 mm / 25 mm 75 mm 12 kN 8 kN/m 75 mm B- 2 m- 4m
-
The beam has a rectangular cross section as shown. Determine the largest intensity w of the uniform distributed load so that the bending stress in the beam does not exceed Ï max = 10 MPa. 50 mm...
-
The beam has the rectangular cross section shown. If w = 1 kN/m, determine the maximum bending stress in the beam. Sketch the stress distribution acting over the cross section. 50 mm I T150 mm -2 2...
-
Required : a- outline the statement of comperhensive income for the year ended 30 november 2021 b- outline the statment of financial position as at 30 November The Trial Balance of Alim Enterprise at...
-
International business and environment The MIR requires teams to gather current, or the most recently available, data on the markets people, economy, government, and technological status from online...
-
Consider the following stream of cash flows. The interest rate is 10%. 0 1 2 3 4 5 6 7 100 100 100 200 0 300 300 300 a) What is the value at time 0 of the cash flow stream? b) What is the value of...

Study smarter with the SolutionInn App


