Write the expression for the equilibrium constant (K p ) for the following: (3)OS = (3)OS +
Question:
Write the expression for the equilibrium constant (Kp) for the following: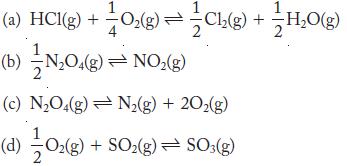
Transcribed Image Text:
(3)OS = (3)OS + (3)0 (P) (c) NO4(g) = N(g) + 20(g) (*)ON (8)0N (9) 30H + (9407 = 90 + (91H ()
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
a K c Kp Cl HO ...View the full answer

Answered By

Aysha Ali
my name is ayesha ali. i have done my matriculation in science topics with a+ . then i got admission in the field of computer science and technology in punjab college, lahore. i have passed my final examination of college with a+ also. after that, i got admission in the biggest university of pakistan which is university of the punjab. i am studying business and information technology in my university. i always stand first in my class. i am very brilliant client. my experts always appreciate my work. my projects are very popular in my university because i always complete my work with extreme devotion. i have a great knowledge about all major science topics. science topics always remain my favorite topics. i am also a home expert. i teach many clients at my home ranging from pre-school level to university level. my clients always show excellent result. i am expert in writing essays, reports, speeches, researches and all type of projects. i also have a vast knowledge about business, marketing, cost accounting and finance. i am also expert in making presentations on powerpoint and microsoft word. if you need any sort of help in any topic, please dont hesitate to consult with me. i will provide you the best work at a very reasonable price. i am quality oriented and i have 5 year experience in the following field.
matriculation in science topics; inter in computer science; bachelors in business and information technology
_embed src=http://www.clocklink.com/clocks/0018-orange.swf?timezone=usa_albany& width=200 height=200 wmode=transparent type=application/x-shockwave-flash_
4.40+
11+ Reviews
14+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Write the expression for the thermodynamic equilibrium constant for each of the following reactions. a. b. c. CO(g) 2H2(g) CH,OHg) 2Ag (a CrO4 (aq)Ag CrO4(s) CaCO3(s) +2H (a)Ca2 (aH20CO2(8)
-
Write the expression for the equilibrium constant Kc for each of the following equations: a. C(s) CO2(g) 2CO(g) b. FeO(s) +CO(g) Fe(s) CO2(g) c. Na2CO3(s) SO2(g) 02(8)Na SO4(s)CO2(g) d. Pbl2(s) Pb...
-
Is this a confined or unconfined aquifer? Please explain. 100 m amsl 78 m amsl 56 m amsl 48 m amsl A 50 m 11m B Clay Sand Clay 100 m
-
Describe three different versions of back flush costing.
-
The unadjusted trial balance of PS Music as of July 31, 2012, along with the adjustment data for the two months ended July 31, 2012, are shown in Chapter 3. Based upon the adjustment data, the...
-
Chinas middle class is about 300 million strong and is growing. How is this important for Alibabas success? How can the company take full advantage of this demographic phenomenon? Appendix
-
In 1950, J. R. Clarkson founded a family-owned industrial valve design and manufacturing company in Sparks, Nevada. For almost a half century, the company, known as the Clarkson Company, worked on...
-
Quilcene Oysteria farms and sells oysters in the Pacific Northwest. The company harvested and sold 7,200 pounds of oysters in August. The company's flexible budget for August appears below: Quilcene...
-
Write the expression for the equilibrium constant (K p ) for the following: (a) Cl(g) + HO(g) 2HCl(g) + 0(g) = (b) 2NO(g) NO4(g) (c) 302(g) 203(g) (d) CO(g) CO(g) +0(g) 2
-
Write the expression for the equilibrium constant (K c ) for the following: (a) 2HO(g) 2H(g) + O(g) (b) 2HCl(g) H(g) + Cl(g) (c) CO(g) + Cl(g) = COC1(g) (d) 2CO(g) + O2(g) 2CO(g)
-
Vischten Co. recently organized. The company issued no-par common stock to an attorney in exchange for his patent with a market value of $64,000. In addition, Vischten Co. received cash for 5,000...
-
Reflect on your semester. How do you plan onmeasuringyour professionalgrowth in the future? What were the most challenging topics to you? What topics felt more intuitive/easy? How do you plan on...
-
Aside from shareholders, who do you believe is the second stakeholder in whose interests the company should be concerned? Justify your response What will you do to ensure the company's success...
-
a) What CSR did your organization do - how did it improve your organization's image? b) If your organization did not do any CSR, as the boss, what CSR activities would you suggest doing and why?
-
Do you believe NIL promotes "love of the game," or does it make college sports more about money and business? What are the most significant positive and negative effects of NIL, in your opinion? What...
-
Even well-managed organizations do not always work as efficiently and effectively as management would like. At Hewlett-Packard (HP), billions of dollars of product are being shipped - from computers...
-
Write a balanced chemical equation using condensed structural formulas for (a) the formation of butyl propionate from the appropriate acid and alcohol, (b) the saponification (base hydrolysis) of...
-
Discuss the concept of the looking-glass self. how do you think others perceive you? do you think most people perceive you correctly?
-
Consider a marble falling through a very thick fluid, such as molasses. Draw qualitative plots of the position, velocity, and acceleration as functions of time for the marble, assuming it is released...
-
A hockey puck that is sliding on an icy surface will eventually come to rest. The (horizontal) force that makes it stop is due to friction between the puck and the ice. Draw qualitative plots of the...
-
Abracadabra! A magician pulls a tablecloth off of a set table with one swift, graceful motion. Amazingly, the fine china, glassware, and silverware are practically undisturbed. Although amazing, this...
-
THIS IS ONE QUESTION WITH TWO PARTS. PLEASE ANSWER COMPLETELY AND SHOW ALL WORK. (NO EXCEL) Information for Question 1: State Probability Retum on A Return on B Return on C Retum on Portfolio X Boom...
-
Direct materials (5.0 Ibs. @ $5.00 per Ib.) Direct labor (2.0 hrs. @ $13.00 per hr.) Overhead (2.0 hrs. @ $18.50 per hr.) Total standard cost $25.00 26.00 37.00 $88.00 The predetermined overhead rate...
-
Problem 1-28 (Algo) (LO 1-4, 1-5, 1-6b 1-7) Harper, Inc., acquires 40 percent of the outstanding voting stock of Kinman Company on January 1, 2020, for $316,100 in cash. The book value of Kinman's...

Study smarter with the SolutionInn App


